Figures & data
Table 1. The table describes different types of paediatric and adult brain tumours both benign and malignant, their location, treatability, age group, and mutations observed in those types of brain tumours.
Figure 1. Figure 1 describes the regions of brain and their functions. (a) Upper Brain, (b) Mid-Brain, and (c) Lower Brain.
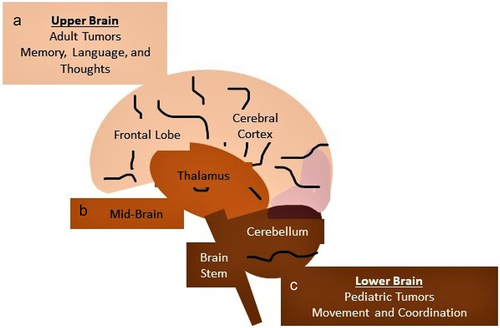
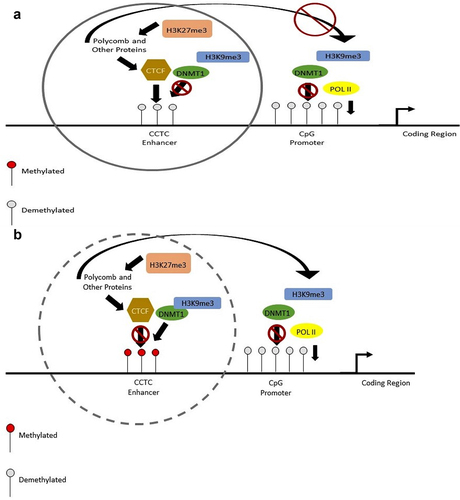
Figure 2. This diagram shows the change in methylation at the enhancer and the promoter regions. With no methylation at the enhancer region, the folding will not occur. (a, c). This will limit the amount transcribed (a), with methylation at the enhancer CCCTC motif, CTCF protein is prevented from binding and the enhancer region will fold over to the promoter to encourage transcription (b, d). Methylation by DNMT1 at the promoter region prevents transcription by not allowing POL II to bind to the region (c, d). When the promoter is not methylated, POL II is able to bind to the sequence and transcribe the genes (a, b).
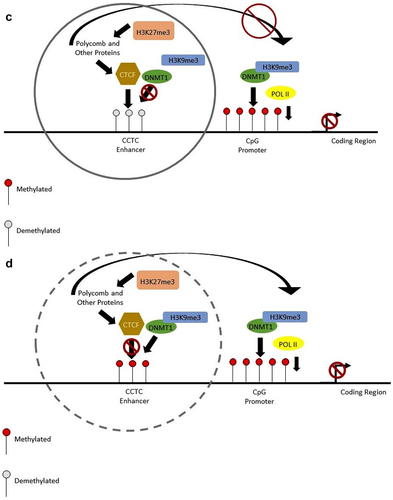
Figure 3. (a) Pediatric Brain Tumour Development. (I) Pluripotent Brain Cells. (II) Differentiation of cells to many specific types of brain cells, (III) One differentiated cells becomes a cancer progenitor cell, and (IV) Rapid growth of this cancer progenitor cell becomes a full-fledged tumour. (b) Adult Brain Tumour Development. (I) Normal differentiated adult cells. (II) One of the differentiated cells become cancer progenitor cells, (III) Rapid growth forming a group of cancer cells, and (IV) Brain tumour including heterogenous call population formed. EMT-epithelial mesenchymal transition, MET-mesenchymal epithelial transition.
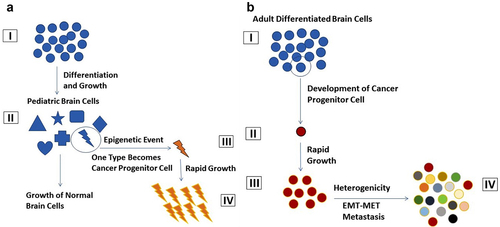
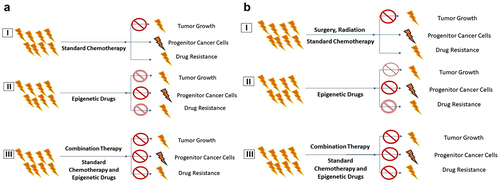
Figure 4. Comparison of Standard Chemotherapy with Combination Therapy. a) Standard Chemo Therapy. (I) Standard chemotherapy, (II) Epigenetic Drug therapy, and (III) Combination therapy with epigenetic Drugs. b) Combination Therapy (I) Surgery, Radiation, Chemo Therapy, Standard therapy, (II) Epigenetic Drug therapy, and (III) Combination therapy with Epigenetic Drugs.