Figures & data
Table 1. Autophagic cargos and/or receptors have been identified and characterized for selective autophagy in plants
Figure 1. Cytoplasmic soluble GFP protein is nonselectively engulfed by autophagosomes and delivered into vacuole upon autophagy induction condition
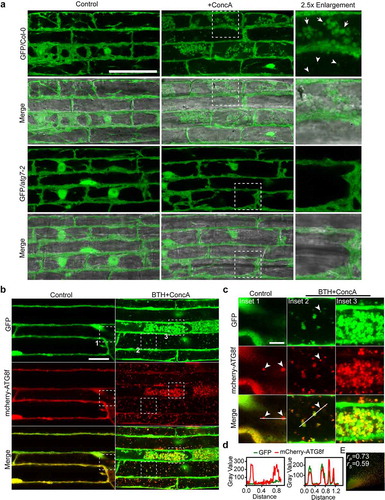
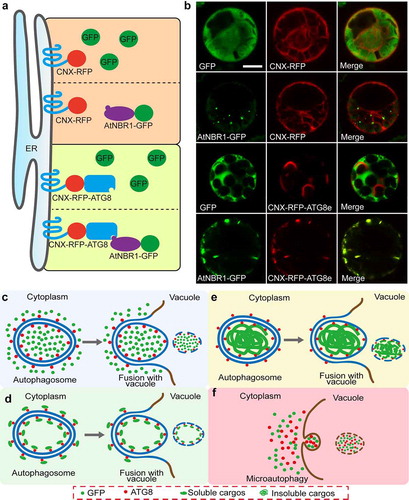
Figure 2. Proposed model for the nonselective cytoplasmic soluble protein and the selective cargos engulfed by autophagosomes