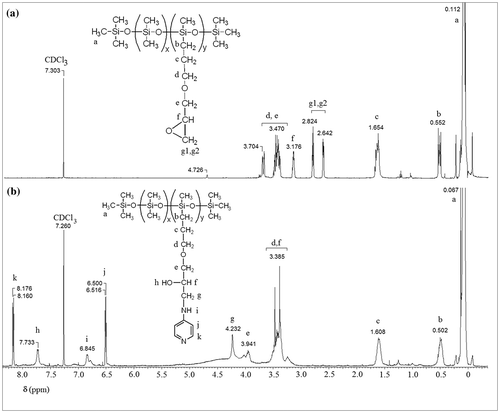
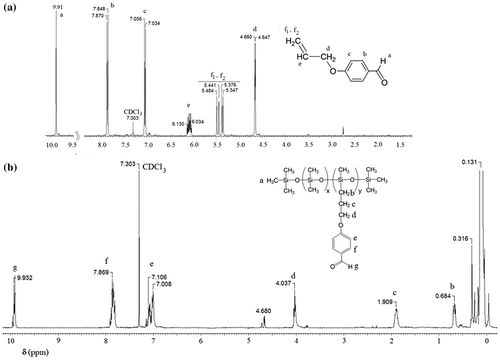
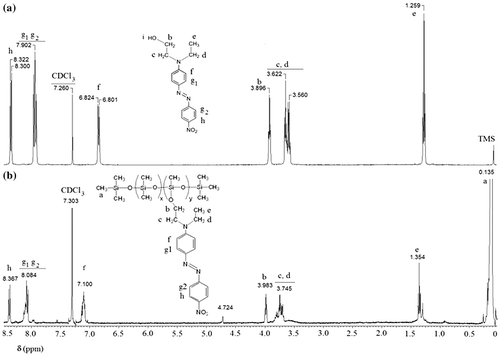
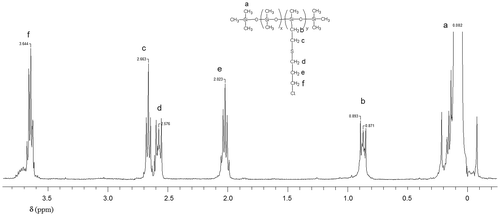
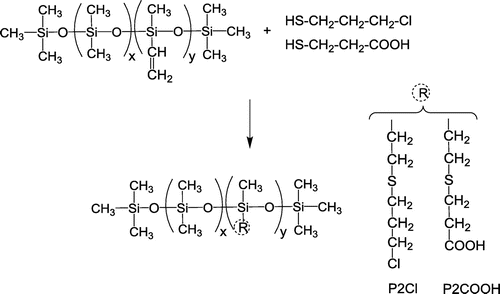
Figures & data
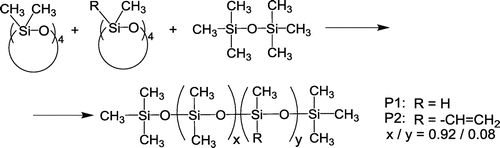
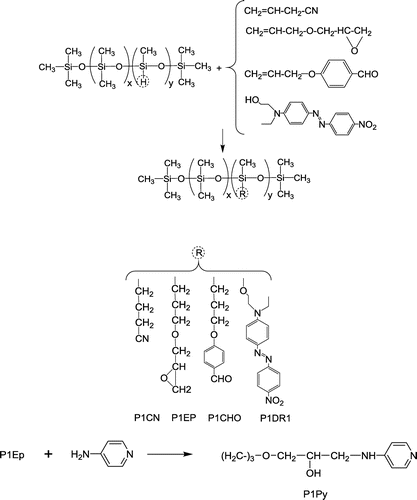
Table 1. Experimental conditions for synthesis of polar silicones.
Figure 6. Dielectric properties of a series of siloxane copolymers having around 8% different polar groups.
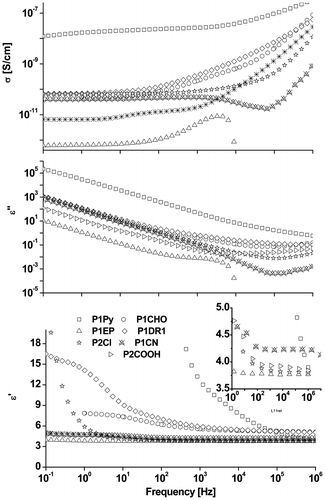
Table 2. Dielectric permittivity, loss, and conductivity (at 10 kHz) of polysiloxanes with different dipoles attached in similar proportion (ca. 8%).
Table 3. Dipole moments, molar polarizability, and molar volume of model molecules containing the polar substituents attached to the silicon atom and a methyl group instead of the polysiloxane chain.
Figure 7. Dielectric permittivity of polar silicones versus a calculated parameter [K1 = (dipol moment) × (molar volume) × (molar polarizability)] of different polar substituents (a) and versus a semi-empirical parameter K2 (b) or K2′ (c), which takes into account the measured moisture sorption capacity, expressed as maximum percents in K2 and as percents for RH = 50% in K2′; K2 = K1 × (% water adsorbed).
![Figure 7. Dielectric permittivity of polar silicones versus a calculated parameter [K1 = (dipol moment) × (molar volume) × (molar polarizability)] of different polar substituents (a) and versus a semi-empirical parameter K2 (b) or K2′ (c), which takes into account the measured moisture sorption capacity, expressed as maximum percents in K2 and as percents for RH = 50% in K2′; K2 = K1 × (% water adsorbed).](/cms/asset/28863711-f34d-459e-9c01-fdc6e1792b12/tdmp_a_1169381_f0007_oc.gif)