Figures & data
Figure 1. Scheme of the protocol. NDMM: newly diagnosed multiple myeloma, BM: bone marrow, VCD: bortezomib-cyclophosphamide-dexamethasone, s.c.: subcutaneous, p.o.: per os, SD: stable disease, G-CSF: granulocyte colony-stimulating factor, ASCT: autologous stem cell transplantation.
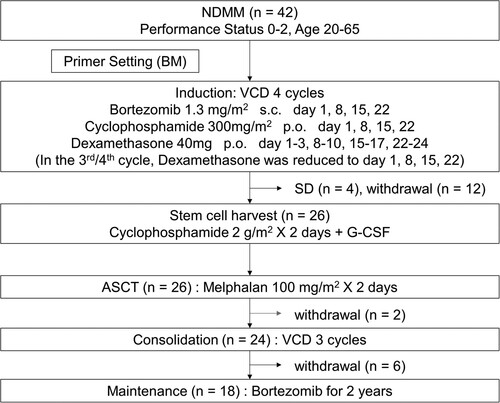
Table 1. Characteristics of the patients.
Table 2. Grade 3/4 adverse events.
Figure 2. Changes in the responses after the treatments. Responses after induction, ASCT and consolidation, and maximum responses after transplantation. sCR: stringent complete response, CR: complete response, VGPR: very good partial response, PR: partial response, SD: stable disease, PD: progressive disease, ASCT: autologous stem cell transplantation.
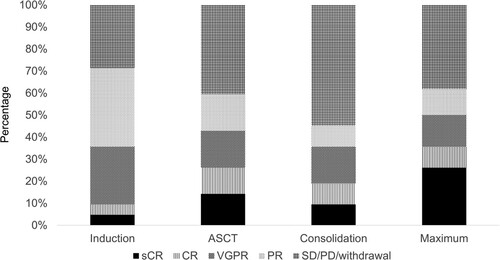
Figure 3. Survival and time to next treatment. (A) Overall survival, (B) Progression-free survival, and (C) Time to next treatment of all patients.
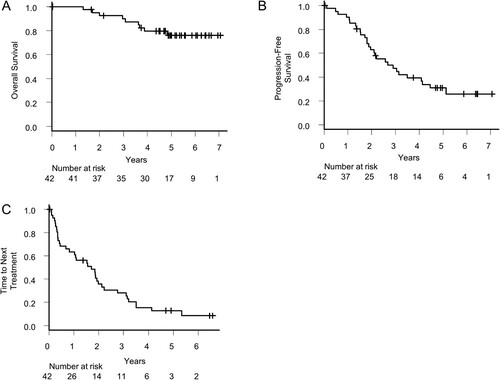
Figure 4. Comparison of survivals by chromosomal abnormalities and response after transplantation. (A) Overall survival and (B) Progression-free survival of the patients with or without high-risk chromosomal abnormalities. (C) Overall survival and (D) Progression-free survival of the patients whose stages in international staging systems were I/II or III. (E) Overall survival and (F) Progression-free survival of the patients who achieved complete response after transplantation or not.
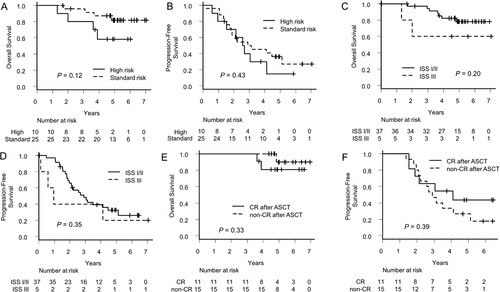
Table 3. Previously reported VCD induction trials.
Supplemental Material
Download TIFF Image (573.3 KB)Data availability statement
The data that support the findings of the present study are available from the corresponding author on reasonable request.
