Figures & data
Figure 1. Study Design. a9 µg/day in cycle 1 (days 1 to 7). bSafety follow-up visit at 30 days (± 3 days) after protocol-specific therapy; thereafter long-term follow-up was every 3 months (± 1 month). alloHSCT, allogeneic hematopoietic stem cell transplantation; CIVI, continuous intravenous infusion; CR, complete remission (CR was defined as ≤ 5% bone marrow blasts, no evidence of disease and full recovery of peripheral blood counts defined as platelets > 100,000/µL and absolute neutrophil count [ANC] > 1000/µL); CRh, complete remission with partial recovery of peripheral blood counts (≤ 5% bone marrow blasts, no evidence of disease, platelets > 50,000/ µL and ANC > 500/µL); CRi, complete remission with incomplete hematological recovery (≤ 5% bone marrow blasts, no evidence of disease, platelets > 100,000/µL or ANC > 1000/µL).
![Figure 1. Study Design. a9 µg/day in cycle 1 (days 1 to 7). bSafety follow-up visit at 30 days (± 3 days) after protocol-specific therapy; thereafter long-term follow-up was every 3 months (± 1 month). alloHSCT, allogeneic hematopoietic stem cell transplantation; CIVI, continuous intravenous infusion; CR, complete remission (CR was defined as ≤ 5% bone marrow blasts, no evidence of disease and full recovery of peripheral blood counts defined as platelets > 100,000/µL and absolute neutrophil count [ANC] > 1000/µL); CRh, complete remission with partial recovery of peripheral blood counts (≤ 5% bone marrow blasts, no evidence of disease, platelets > 50,000/ µL and ANC > 500/µL); CRi, complete remission with incomplete hematological recovery (≤ 5% bone marrow blasts, no evidence of disease, platelets > 100,000/µL or ANC > 1000/µL).](/cms/asset/ee5867e0-f0d4-46ec-9f4d-1572be1408dc/yhem_a_2111992_f0001_oc.jpg)
Table 1. Demographics and Baseline Characteristics.
Table 2. Best Response During First 2 Cycles of Blinatumomab.
Figure 2. Forest Plot for CR/CRh During First 2 Cycles of Blinatumomab. The CR/CRh rate during the first 2 cycles of blinatumomab was compared within subgroups (sex [female vs male], age [< 35 vs ≥ 35 to < 55 vs ≥ 55 years], prior salvage therapy [yes vs no], prior alloHSCT [yes vs no], bone marrow blasts at baseline [< 50% vs ≥ 50%], disease status [primary refractory vs first relapse vs second or later relapse], and refractory to last previous therapy [yes vs no]). Logistic regression was used to estimate the odds ratio and its 95% CIs between subgroups. alloHSCT, allogeneic hematopoietic stem cell transplantation; CI, confidence interval; CR, complete remission; CRh, complete remission with partial hematological recovery.
![Figure 2. Forest Plot for CR/CRh During First 2 Cycles of Blinatumomab. The CR/CRh rate during the first 2 cycles of blinatumomab was compared within subgroups (sex [female vs male], age [< 35 vs ≥ 35 to < 55 vs ≥ 55 years], prior salvage therapy [yes vs no], prior alloHSCT [yes vs no], bone marrow blasts at baseline [< 50% vs ≥ 50%], disease status [primary refractory vs first relapse vs second or later relapse], and refractory to last previous therapy [yes vs no]). Logistic regression was used to estimate the odds ratio and its 95% CIs between subgroups. alloHSCT, allogeneic hematopoietic stem cell transplantation; CI, confidence interval; CR, complete remission; CRh, complete remission with partial hematological recovery.](/cms/asset/174724d7-6792-4c12-868e-f67d3feb9c16/yhem_a_2111992_f0002_oc.jpg)
Figure 3. Overall Survival. Overall survival time was calculated from the time of first infusion of blinatumomab until death due to any cause. Patients still alive were censored at the date last known to be alive until the data cutoff date of April 12, 2019. Months are calculated as days from the first treatment to death/censor date, divided by 30.5. CI, confidence interval.
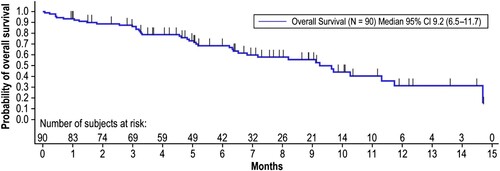
Figure 4. Relapse-Free Survival. Relapse-free survival time was calculated from the first onset of CR/CRh within the 2 cycles of blinatumomab until documented hematological relapse, extramedullary disease, or death due to any cause, whichever occurred first. Patients still alive and relapse-free were censored at the date of last disease assessment up until the data cutoff date of April 12, 2019. Months are calculated as days from the first onset of CR/CRh within the 2 cycles of blinatumomab until documented hematological relapse/extramedullary disease/death/censor date, divided by 30.5. CI, confidence interval; CR, complete remission; CRh, complete remission with partial hematological recovery.
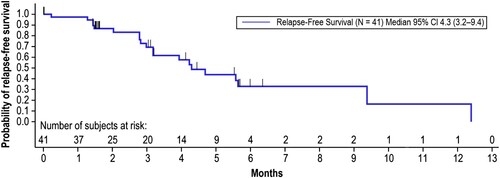
Table 3. Proportion of Patients Undergoing alloHSCT.
Figure 5. Pharmacokinetics of Blinatumomab in Adults With R/R ALL From China, Japan, and Globally. Css was determined after 5 half-lives from start of continuous IV infusion of 9 and 28 μg/day blinatumomab to adult patients in cycle 1 for the Chinese cohort, and compared with the Japanese [Citation21] and global cohorts (Amgen data on file, refer to Data Sharing Statement). Boxes display mean (dashed lines), median (solid lines), 25th (bottom) percentile, and 75th (top) percentile. Whiskers represent the 10th (bottom) and 90th (top) percentiles. ALL, acute lymphoblastic leukemia; Css, concentration at steady-state; IV, intravenous; R/R, relapsed/refractory.
![Figure 5. Pharmacokinetics of Blinatumomab in Adults With R/R ALL From China, Japan, and Globally. Css was determined after 5 half-lives from start of continuous IV infusion of 9 and 28 μg/day blinatumomab to adult patients in cycle 1 for the Chinese cohort, and compared with the Japanese [Citation21] and global cohorts (Amgen data on file, refer to Data Sharing Statement). Boxes display mean (dashed lines), median (solid lines), 25th (bottom) percentile, and 75th (top) percentile. Whiskers represent the 10th (bottom) and 90th (top) percentiles. ALL, acute lymphoblastic leukemia; Css, concentration at steady-state; IV, intravenous; R/R, relapsed/refractory.](/cms/asset/6b2f66bc-f79d-4730-9ba5-0993558148cb/yhem_a_2111992_f0005_ob.jpg)
Table 4. Comparison of Demographics and Baseline Characteristics, Pharmacokinetics, and Treatment-Emergent Adverse Events of Blinatumomab in Adults With R/R ALL From China, Japan, and Globally.
Data availability statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing
