Figures & data
Figure 1. Patient flow chart. aEthical considerations precluded capture of reasons for voluntary discontinuation and any further data on respondents who quit (see Methods, Ethics Statements and Data Confidentiality).
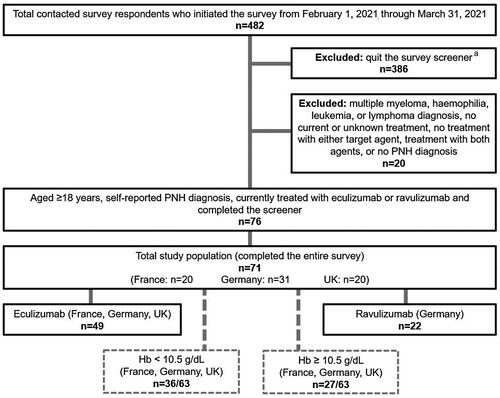
Table 1. Patient characteristics.
Table 2. Dosage.
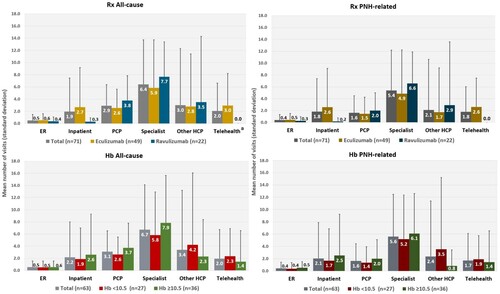
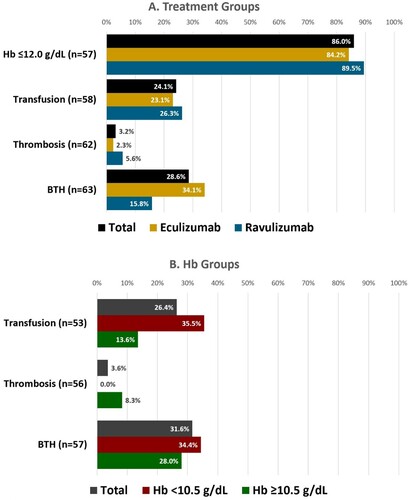
Figure 2. Haematological outcomes. BTH, breakthrough haemolysis; Hb, (serum) hemoglobin.
Notes: Data reflect events in the past 12 months, among query respondents treated for ≥12 months. Breakthrough haemolysis was defined in the survey as: ‘ … the return of haemolytic disease activity e.g. return of (my) symptoms/ anaemia / major vascular event e.g. a blood clot.’
Supplemental Material
Download MS Word (87 KB)Supplemental Material
Download MS Word (87 KB)Data availability
Anonymised patient source data are not available; all relevant analysed data are included in the manuscript and supplementary materials.
Data availability
Anonymised patient source data are not available; all relevant analysed data are included in the manuscript and supplementary materials.