Figures & data
Figure 1. Schemes of chemoimmunotherapy prior to autologous transplant. V = Bortezomib. R = Lenalidomide. T = Thalidomide. D = Dexamethasone. PACE = Cisplatin/doxorubicin/cyclophosphamide/etoposide.
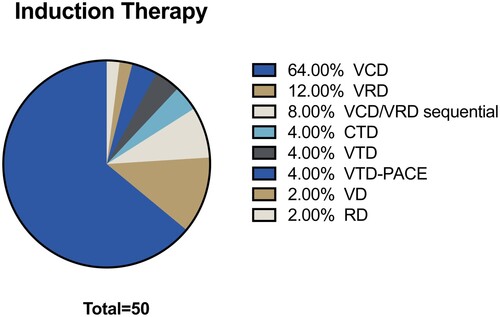
Table 1. Characteristics of patients undergoing autologous stem cell transplantation (ASCT).
Figure 2. Response criteria for patients with multiple myeloma before and after autologous transplant. VGPR: very good partial response. sCR: strict complete response. PD: disease progression. AutoHSCT: autologous hematopoietic stem cell transplantation.
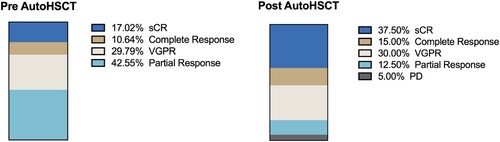
Figure 3. Progression-free survival and overall survival in patients with multiple myeloma undergoing autologous hematopoietic stem cell transplantation.
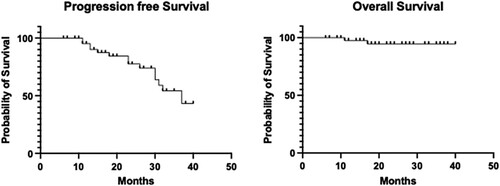
Figure 4. Progression-free survival in patients with multiple myeloma undergoing autologous hematopoietic stem cell transplant according to pre-transplant (A), post-transplant response criteria (B–C) and measurable residual disease post-transplant (D).
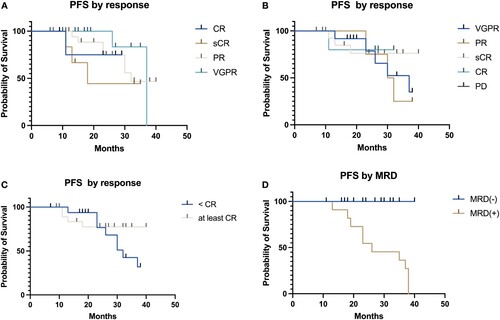
Table 2. Adverse events reported in patients undergoing autologous transplant.
