Figures & data
Figure 1. Suppression by rosmarinic acid (RA) of peroxisome proliferator-activated receptor-γ (PPARγ) and CCAAT/enhancer binding protein-α (C/EBPα) protein expression during the differentiation of 3T3-L1 adipocytes. Cells were collected on days 3, 6, and 9 during differentiation for the measurement of PPARγ and C/EBPα protein expression by Western blotting. Representative blots are shown above the graphs. Data are presented as means ± SEM. All cell culture experiments were repeated at least three times on two separate passages of cells (n = 6). *p < 0.05 vs vehicle (Veh) group at the same time-point; #p < 0.05 vs RA group on day 3 of differentiation.
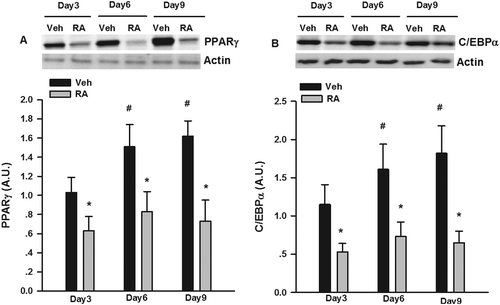
Figure 2. Inhibition of adipogenesis by rosmarinic acid (RA) via the activation of extracellular signal-regulated kinase-1/2 (ERK1/2) and mothers against decapentaplegic homolog 3 (Smad3). (A) Protein expression of phosphorylated (p-)ERK1/2 and Smad3 after RA (50 μM, 12 h) incubation on day 6 of differentiation. (B) Oil Red O staining of the 3T3-L1 adipocytes (on day 6 post-differentiation) treated with RA in the absence or presence of PD98059 (PD, 25 μM) and SB431542 (SB, 2 μM) for 24 h. (C) Protein expression of p-ERK1/2 and Smad3 after RA (50 μM, 12 h) incubation in the presence or absence of PD98059 (25 μM) and SB431542 (2 μM) for 24 h. Representative blots are shown to the right of the graphs. Data are presented as means ± SEM in (A) and (C). All cell culture experiments were repeated at least three times on two separate passages of cells (n = 6). *p < 0.05 vs vehicle (Veh) group.
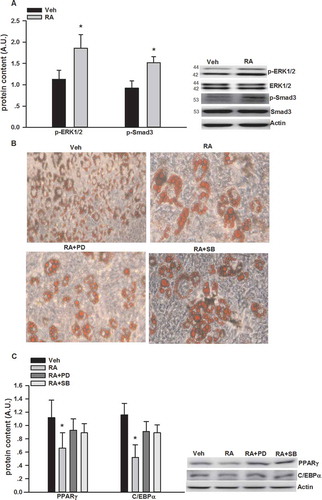
Table 1. Effects of rosmarinic acid (RA) on basal glycerol and free fatty acid (FFA) release (μm/L).
Figure 3. Glycerol and free fatty acid (FFA) release in cultured 3T3-L1 adipocytes incubated with rosmarinic acid (RA) in the presence or absence of wortmannin (wort, 100 nM), rolipram (rol, 5 μM), and cilostamide (cil, 5 μM) in Dulbecco’s modified Eagle’s medium supplemented with 2% fatty acid-free bovine serum albumin at 2 h (A), 24 h (B), and 48 h (C). Data are presented as means ± SEM. All cell culture experiments were repeated at least three times on two separate passages of cells (n = 6). *p < 0.05 vs vehicle (Veh) group at the same time-point; #p < 0.05 vs RA group at the same time-point.
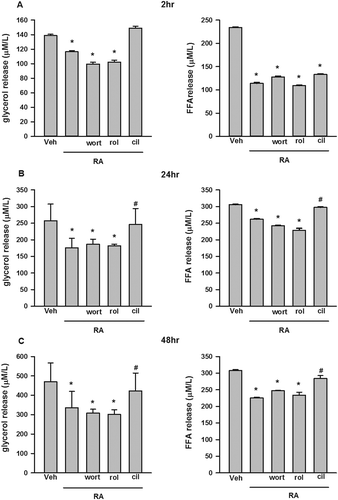
Figure 4. Inhibition by rosmarinic acid (RA) of isoproterenol (ISO)- and forskolin (forsk)- stimulated glycerol and free fatty acid (FFA) release in cultured 3T3-L1 adipocytes. RA suppressed ISO- and forsk-stimulated glycerol and FFA release at both 2 h (A) and 24 h (B). RA inhibited ISO- and forsk-induced phosphorylation of hormone-sensitive lipase (HSL)ser660 and perilipin A (C). Representative blots are shown to the right of the graphs in (C). Data are presented as means ± SEM. All cell culture experiments were repeated at least three times on two separate passages of cells (n = 6). *p < 0.05 vs vehicle (Veh) group at the same time-point; #p < 0.05 vs RA group at the same time-point.
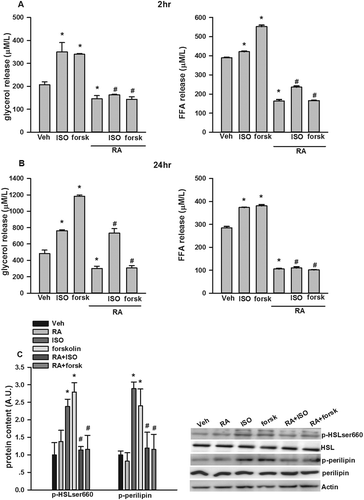
Figure 5. Suppression by rosmarinic acid (RA) of lipopolysaccharide (LPS)-stimulated tumor necrosis factor-α (TNF-α) messenger RNA (mRNA) expression and secretion in RAW 264.7 macrophages, and inflammatory cytokine mRNA expression in 3T3-L1 adipocytes stimulated with LPS-macrophage-conditioned medium (MCM). RA inhibited LPS-stimulated TNF-α mRNA expression (A) and secretion (B) in RAW 264.7 macrophages. RA inhibited interleukin-6 (IL-6), IL-1β, monocyte chemoattractant protein-1 (MCP-1), and regulated on activation, normal T cell expressed and secreted (RANTES) mRNA expression in 3T3-L1 adipocytes incubated with LPS-MCM (C). *p < 0.05 vs vehicle (Veh) group; #p < 0.05 vs LPS group (in A and B); #p < 0.05 vs LPS-MCM group (in C).
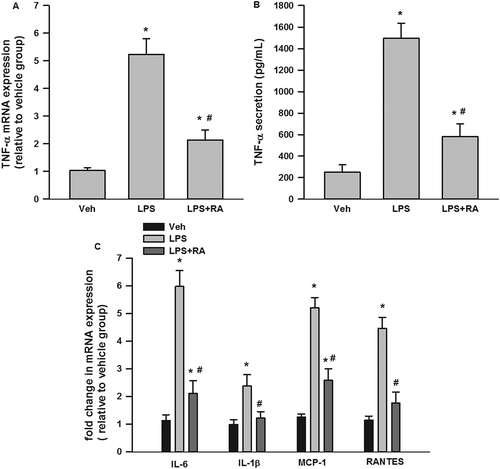
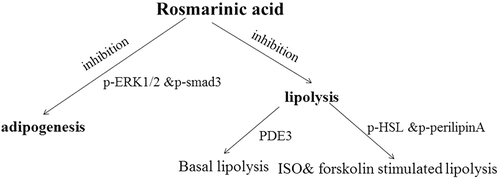
Figure 6. Brief summary of the metabolic networks involved in the effects of rosmarinic acid (RA) on adipogenesis and lipolysis. RA inhibits adipogenesis via phosphorylated extracellular signal-regulated kinase-1/2 (p-ERK1/2) and mothers against decapentaplegic homolog 3 (p-Smad3), basal lipolysis via phosphodiesterase-3 (PDE3), and isoproterenol (ISO)- and forskolin-stimulated lipolysis via hormone-sensitive lipase (p-HSL) and p-perilipin A.