Figures & data
Figure 1. In vitro delivered fine particle doses as percent of label claim (FPFs < 5 µm) from various DPIs tested at 4 kPa (high resistance devices) or 2 kPa (low resistance devices): range of flow rates 40–75 L/min using a Next Generation Impactor. Data derived from different comparative in vitro evaluation studies performed by the authors at the same conditions for direct comparision. Grey bars are for inhalers carrying adhesive mixtures; dark grey bars with force control agents in the formulation. Shaded bars are for carrier free (Turbuhaler) formulations. FPFs are for budesonide (Easyhaler, Cyclohaler, M2 and M3 Turbuhaler and Novolizer), fluticason propionate (Diskus and Elpenhaler), beclometason dipropionate (NEXThaler), aclidinium bromide (Genuair), tiotropium bromide (Handihaler), indacaterol maleate (Breezhaler) and disodium cromoglycate (Spinhaler).
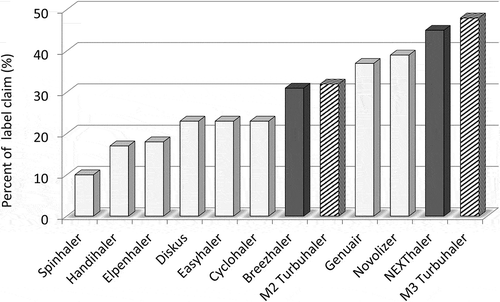
Figure 2. Fine particle fraction of the delivered dose and part of the delivered dose deposited in the mouth and oropharynx from DPIs carrying adhesive mixtures as drug formulation. A: current situation. The fraction not released from carrier and the fine particle fraction were averaged for the DPIs presented in and the oropharyngeal losses were estimated from in vivo deposition studies with radiolabel drugs in various marketed DPIs. B: desired situation to be obtained by improved dispersion (from 35% to 65% drug detachment) at a lower flow rate (from ≫ 60 L/min to < 40 L/min).
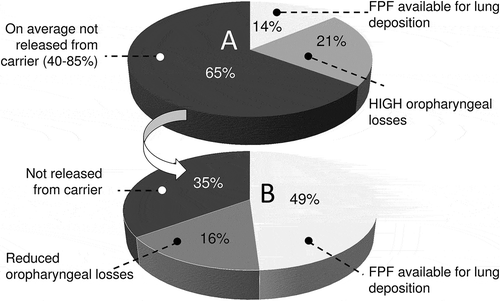
Figure 3. The oral cavity during DPI use of a child. a: Captures from a video recording of the changes in the passageway for the aerosol during a single inhalation manoeuvre; b; examples of a narrowed passageway by elevated tongue and inwards directed displacement of the cheeks (captures from different video recordings of different children showing some extreme situations). The recordings were made using a bronchoscope in the mouthpiece of a test inhaler with exchangeable mouthpieces and air flow resistances [Citation150].
![Figure 3. The oral cavity during DPI use of a child. a: Captures from a video recording of the changes in the passageway for the aerosol during a single inhalation manoeuvre; b; examples of a narrowed passageway by elevated tongue and inwards directed displacement of the cheeks (captures from different video recordings of different children showing some extreme situations). The recordings were made using a bronchoscope in the mouthpiece of a test inhaler with exchangeable mouthpieces and air flow resistances [Citation150].](/cms/asset/a3b07d4f-e9e4-4a1f-8377-6ac4a2d5cab3/iedd_a_1224846_f0003_oc.jpg)
Table 1. Future challenges and objectives for DPIs.
Figure 4. The desired balance between the interparticulate force in the powder formulation, the dispersion generated by the inhaler and the lung deposition forces during inhalation. Appropriate balancing of these forces is needed for optimal DPI performance.