Figures & data
Figure 3. N2 adsorption and desorption isotherms for the Mn3O4 and Mn3O4.CuO samples (a); pore size distribution of Mn3O4 and Mn3O4.CuO (b).
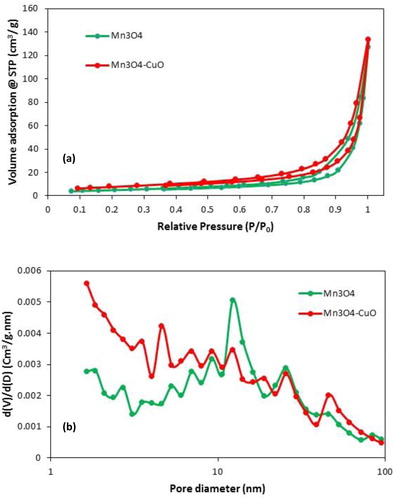
Figure 4. FESEM images of Mn3O4.CuO (a); EDS spectra of Mn3O4.CuO (b); elemental mapping images of Mn3O4.CuO for Mn(c), Cu (d), O (e).
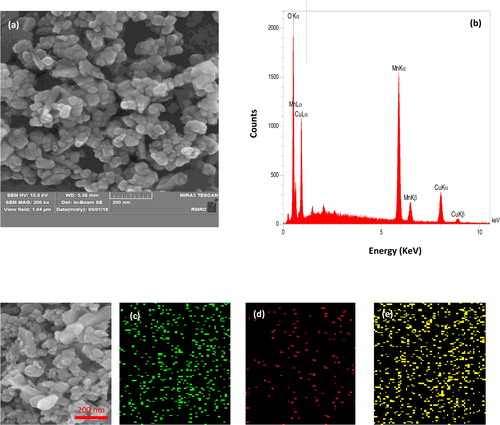
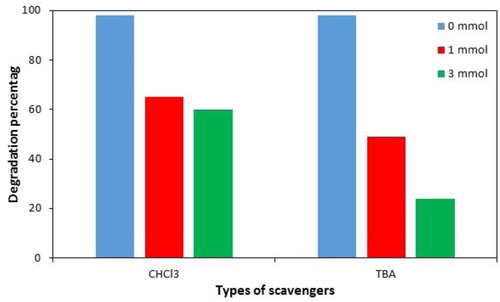
Figure 6. Influence of different operation parameters on PNP degradation. Catalyst mass (a) (experimental conditions: initial concentration of H2O2: 0.1 mol L-1, sonication time: 20 min and pH: 8.0); H2O2 concentration (b) (experimental conditions: catalyst mass: 0.015 g, sonication time: 20 min and pH: 8.0); pH (c) (experimental conditions: catalyst mass: 0.015 g, sonication time: 20 min and initial concentration of H2O2: 0.1 mol L-1); Sonication time (d) (experimental conditions: catalyst mass: 0.015 g, initial concentration of H2O2: 0.1 mol L-1 and pH: 8.0).
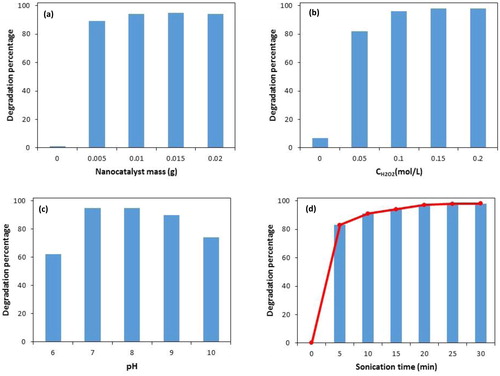
Table 1. Comparison of the catalytic performance of some reported catalysts with Mn3O4.CuO nanocomposite for ultrasonic degradation of nitrophenol compounds.