Open access
1,416
Views
4
CrossRef citations to date
0
Altmetric
Article
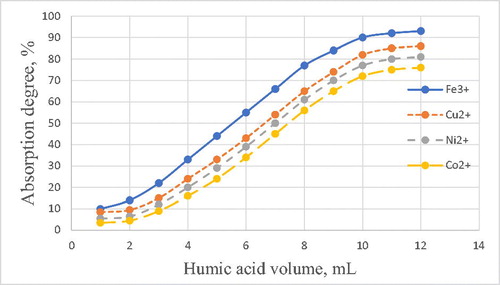
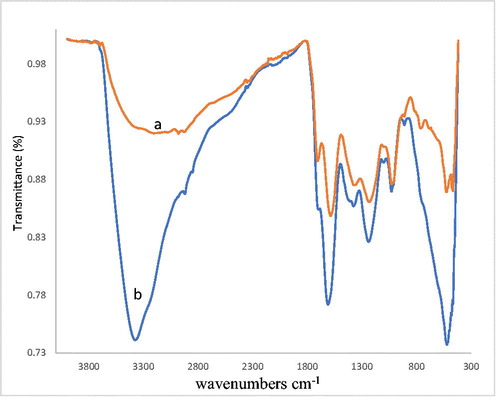
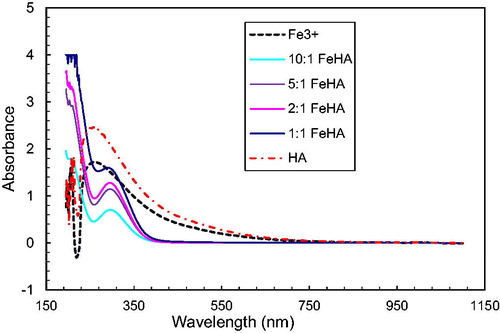
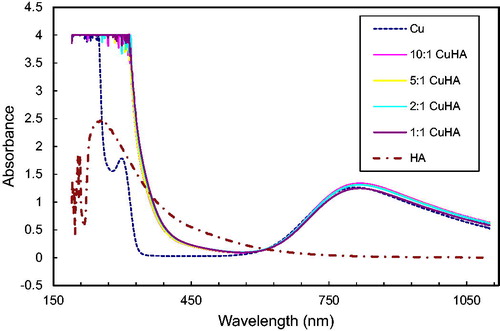
Interaction of metal ions with humic acids of brown coals of Kazakhstan
A. DauletbayChemistry and Chemical Technology, Al-Farabi Kazakh National University, Almaty, KazakhstanCorrespondence[email protected]
 https://orcid.org/0000-0003-0157-1449View further author information
https://orcid.org/0000-0003-0157-1449View further author information
B. A. SerikbayevChemistry and Chemical Technology, Al-Farabi Kazakh National University, Almaty, KazakhstanView further author information
, D. Kh. KamysbayevChemistry and Chemical Technology, Al-Farabi Kazakh National University, Almaty, KazakhstanView further author information
& L. K. KudreevaChemistry and Chemical Technology, Al-Farabi Kazakh National University, Almaty, KazakhstanView further author information
Pages 406-416
|
Received 09 Oct 2019, Accepted 06 Aug 2020, Published online: 25 Aug 2020
Related research
People also read lists articles that other readers of this article have read.
Recommended articles lists articles that we recommend and is powered by our AI driven recommendation engine.
Cited by lists all citing articles based on Crossref citations.
Articles with the Crossref icon will open in a new tab.