Figures & data
Figure 1. Steps involved in countermeasure development following FDA animal rule. Such countermeasures under development may receive pre-emergency use authorization (pre-EUA) prior to full approval for human use by FDA. BLA, biologics license applications; IND, investigational new drug; NDA, new drug application.
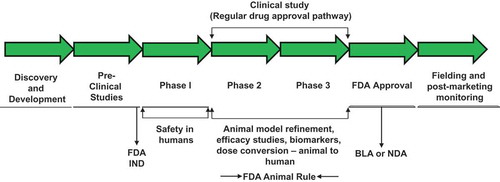
Table 1. U.S. FDA-approved biologics for H-ARS following animal rule and available in SNS/VMI.
Table 2. Radiation countermeasures under development following the FDA animal rule criteria for ARS with U.S. FDA IND status.
