Figures & data
Figure 1. Structure of the Emulate Liver-Chip. The Liver-Chip employs two parallel microfluidic channels (A and H) that are separated by a porous polymer membrane (D) and lined with the major cell types present within the human liver sinusoid. Primary human hepatocytes (C) are situated in the upper channel (A) and sandwiched between two layers of extracellular matrix (B). Liver Sinusoidal Endothelial cells (G), Kupffer cells (F) and stellate cells (E) are cultured on the opposite side of the membrane in the lower channel (H). Figure 1 is original artwork of Emulate Inc. The figure is reproduced from [Citation30] with permission from Emulate Inc.
![Figure 1. Structure of the Emulate Liver-Chip. The Liver-Chip employs two parallel microfluidic channels (A and H) that are separated by a porous polymer membrane (D) and lined with the major cell types present within the human liver sinusoid. Primary human hepatocytes (C) are situated in the upper channel (A) and sandwiched between two layers of extracellular matrix (B). Liver Sinusoidal Endothelial cells (G), Kupffer cells (F) and stellate cells (E) are cultured on the opposite side of the membrane in the lower channel (H). Figure 1 is original artwork of Emulate Inc. The figure is reproduced from [Citation30] with permission from Emulate Inc.](/cms/asset/c87ff192-0fc9-47f8-ae1c-a90ebb0c0e5e/iedc_a_2255127_f0001_oc.jpg)
Figure 2. Workflow for assessing potential DILI severity. when a compound of interest is run through the Liver-Chip, its effects on albumin production, alanine transaminase (ALT) release, and hepatocyte morphology are measured. These readouts are combined with the compound’s human plasma Cmax for efficacy to compute a Liver-Chip DILI score. These scores can then be mapped to industry-standard DILI risk categories to provide the drug developer with information on the potential severity of injury that may be caused by the compound if it progresses into the clinic. Used with permission of Emulate Inc.
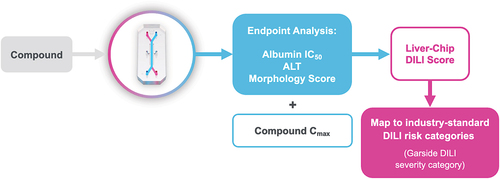
Figure 3. Mapping of Liver-Chip DILI score to DILI severity categories. Liver-Chip DILI scores can be divided into five bands, and these can be mapped to a prediction of DILI severity. Indicated along the columns are Garside severity categories, a standard hepatotoxicity ranking system that is based on clinical observation. As the Liver-Chip DILI score increases, the positive predictive value (PPV) shifts toward the less severe risk categories. Used with permission of Emulate Inc.
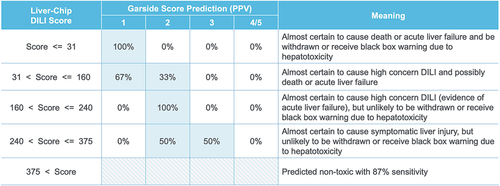
Figure 4. Positioning the Liver-Chip into the drug development workflow to support regulatory submission. Use of the human Liver-Chip after or concurrently with animal good Laboratory Practice (GLP) toxicology studies can provide drug developers with further confidence in the safety profile of a candidate drug prior to entry into clinical trials. Liver-Chip DILI scores obtained in this manner can be integrated with the animal study results using the decision matrix in Figure 5. Alternatively, the Liver-Chip may be used prior to animal testing to help researchers divest from prohibitively toxic compounds before animals are sacrificed for safety testing or to limit the range of doses used in animal experiments. This alternative workflow is consistent with 3 R goals as described in Ewart et al., 2022 [Citation25]. Used with permission of Emulate Inc.
![Figure 4. Positioning the Liver-Chip into the drug development workflow to support regulatory submission. Use of the human Liver-Chip after or concurrently with animal good Laboratory Practice (GLP) toxicology studies can provide drug developers with further confidence in the safety profile of a candidate drug prior to entry into clinical trials. Liver-Chip DILI scores obtained in this manner can be integrated with the animal study results using the decision matrix in Figure 5. Alternatively, the Liver-Chip may be used prior to animal testing to help researchers divest from prohibitively toxic compounds before animals are sacrificed for safety testing or to limit the range of doses used in animal experiments. This alternative workflow is consistent with 3 R goals as described in Ewart et al., 2022 [Citation25]. Used with permission of Emulate Inc.](/cms/asset/ab640b68-ca46-4963-824f-7eea41ce7cea/iedc_a_2255127_f0004_oc.jpg)
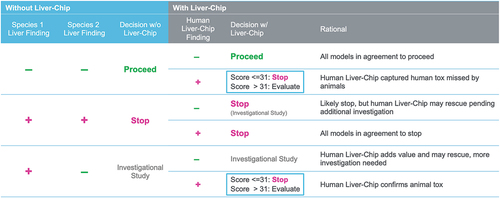
Figure 5. Decision matrix for combining animal model and Liver-Chip data to improve preclinical decision making. This chart maps out the possible scenarios faced by a project toxicologist after performing studies in two animal species and cross-referencing these with possible findings from a human Liver-Chip study. This analysis can furthermore leverage quantitative output from the Liver-Chip as captured by the Liver-Chip DILI score to provide a sense of potential DILI severity, affording greater confidence in the decision to progress a candidate drug into the clinic. Used with permission of Emulate Inc.