Figures & data
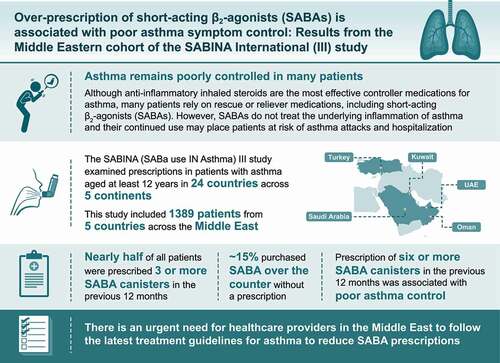
Figure 1. Patient disposition and study population by practice type and investigator-classified asthma severity in the SABINA III Middle East cohort (n=1389). SABINA, SABA use IN Asthma.
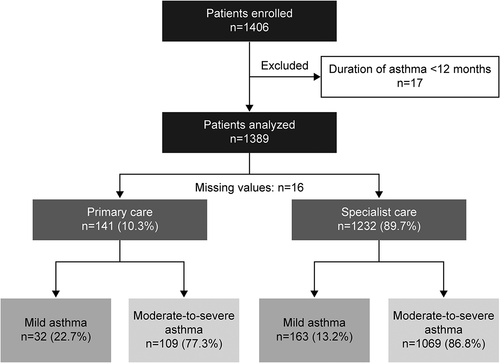
Table 1. Baseline demographics and lifestyle characteristics by investigator-classified asthma severity and practice type in the SABINA III Middle East cohort.
Table 2. Asthma-related clinical characteristics in the SABINA III Middle East cohort.
Figure 2. SABA prescriptions in the 12 months prior to study entry according to investigator-classified asthma severity and practice type in the SABINA III Middle East cohort. SABA, short-acting β2-agonist; SABINA, SABA use IN Asthma. *Patients without SABA prescriptions did not report which reliever they were using.
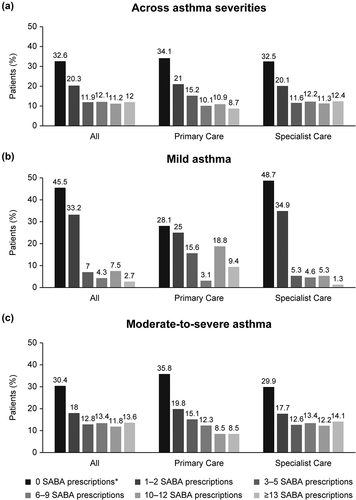
Table 3. SABA prescriptions in the 12 months prior to study entry in the SABINA III Middle East cohort.
Table 4. SABA OTC purchase in the 12 months prior to study entry in the SABINA III Middle East cohort.
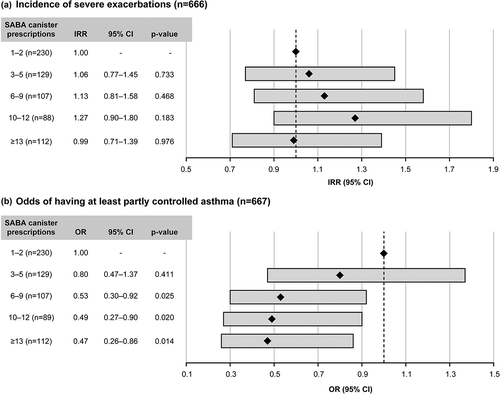
Figure 3. Association of SABA prescriptions with (a) severe exacerbations in the 12 months prior to study entry and (b) level of asthma control assessed during the study visit in the SABINA III Middle East cohort. BMI, body mass index; CI, confidence interval; GINA, Global Initiative for Asthma; IRR, incidence rate ratio; OR, odds ratio; SABA, short-acting β2-agonist; SABINA, SABA use IN Asthma. Based on the covariable significance in the models, IRRs and ORs were corrected by country, age, sex, BMI, smoking, duration of asthma, prescriber type, GINA step by investigator, healthcare insurance, education level, and comorbidities.
Supplemental Material
Download Zip (311.7 KB)Data availability statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
