Abstract
Aim: To synthesize a novel series of norfloxacin analogs and to evaluate biological activity. Methodology: Novel norfloxacin analogs were synthesized and characterized by NMR and mass spectrometry. Antiproliferative and antioxidant properties were studied. Results: Compound 2f was the most potent against HeLa cell-line with 100% inhibition of cell viability IC50 = 3.1 ± 0.2 μM. All compounds exhibit moderate to excellent antioxidant properties. Docking study demonstrates higher binding affinity of compounds with respective anticancer (B-cell lymphoma-2) and (tyrosinase) antioxidant targets. In silico absorption, distribution, metabolism and excretion profile of compounds proves all synthesized compounds follow Lipinski’s rule of drug likeness, non toxic and possess passive gastrointestinal absorption. Conclusion: The biological profile suggest that the synthesized norfloxacin analogs can be a novel scaffold for future anticancer drug development.
Experimental methods
In the current study two novel series of norfloxacin analogs were synthesized by nucleophilic substitution reaction at piperazine ring and structural elucidation of all analogs was confirmed by 1H-NMR, 13C-NMR and mass spectrometry.
Standard MTT calorimetric assay was used to evaluate the cytotoxic potential of compounds and antioxidant properties were examined by different assays like radical scavenging assay, metal chelating, total antioxidant capacity and total reducing power assay.
Results
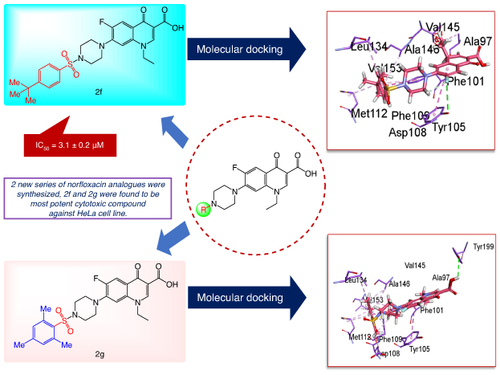
All the synthesized compounds (2a-2h, 3a-3c) demonstrated anticancer activity against the HeLa cell line in the following order 2f > 2g > 2e > 2f > 2d > 2c > 2b > 2h > 3b > 3c > 3a.
Molecular docking data indicates compounds 2f, 2g and 3c exhibit highest binding affinity toward respective protein targets B-cell lymphoma-2 and tyrosinase.
In silico physicochemical data and absorption, distribution, metabolism and excretion profile of synthesized compounds illustrates that all the synthesized compounds were following Lipinski’s rule of drug likeness, nontoxic and possess passive gastrointestinal absorption.
Conclusion
All synthesized analogs possess medium to strong anticancer and antioxidant activities. Compound 2f found to be most potent with cell viability of 100% inhibition with IC50 = 3.1 ± 0.2 μM.
Acknowledgments
Acknowledged to HEJ Research Institute, University of Karachi, Pakistan and COMSATS University, Islamabad, Pakistan for providing excellent research facilities.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.