Figures & data
Figure 1. Scheme of the initial intermolecular contacts leading to amyloid formation according to different models. (A) The amyloid classical model relies on the initial establishment of interactions (thin lines) between short APRs of about 5–10 residues with a predominant hydrophobic character (red boxes). (B) The prion compositional model relies on the initial establishment of a large number of diffuse weak interactions (thin lines) along the PFD. (C) In the new model for prion and prion-like proteins, the first contacts (thin lines) are formed between short amyloidogenic regions (red boxes) embedded into a Q/N-rich disordered region. The amyloid core is longer than those in the classical amyloid model, and, due to their particular composition, display less amyloidogenic potential. This allows the protein to remain soluble until needed and results in assemblies with a significant degree of brittleness.
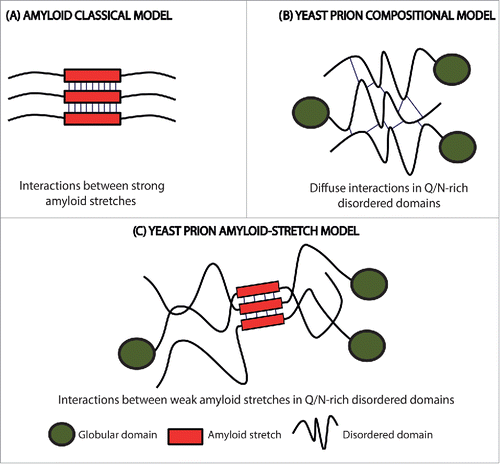
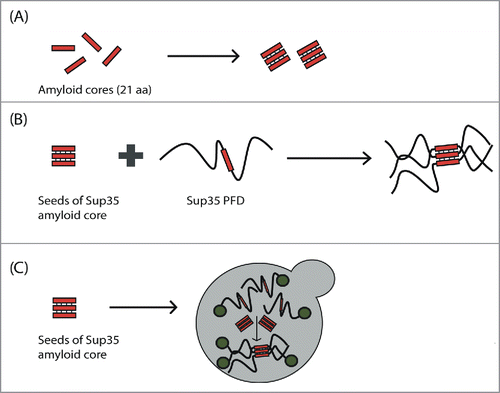
Figure 2. Scheme of the experimental evidence for the presence of amyloid cores in canonical yeast prions. (A) Amyloid cores from Sup35, Ure2, Swi1 and Mot3 are able to make the transition from the soluble and disordered state to amyloid fibrils with cross-β structure. (B) The Sup35 amyloid core is able to promote (seed) the aggregation of the complete Sup35 PFD in vitro. (C) Introduction of Sup35 amyloid core seeds into yeast is able to induce the aggregation of the endogenous Sup35 protein in vivo and, consequently, the expression of the prionic phenotype.