Figures & data
Table 1. General description of the IgGsFootnote
Table 2. Biophysical and cynomolgus monkey FcRn binding properties of the IgGs
Figure 1. CDR charge balancing reduces non-specific binding to heparin. Non-specific binding of (A) A and B and (B) C and D to heparin-coated plates.
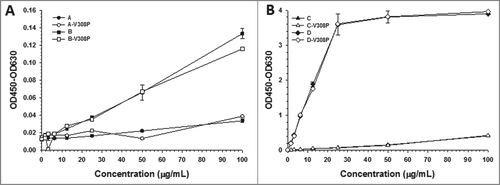
Figure 2. CDR charge balancing improves the cynomolgus monkey pharmacokinetics of mAbs. Pharmacokinetic profiles of (A) mAbs A and B and (B) C and D. mAbs A and C are CDR charge balanced from mAbs B and D, respectively. Data are the mean of 2 animals/time point for mAb D. Data are the mean ± SD (standard deviation) for 4 animals/time point for mAb C and 3 animals/time point for mAbs A and B. The pharmacokinetics were assessed following a single IV dose of 2 mg/kg for mAbs A and B and 10 mg/kg for mAb C and D.
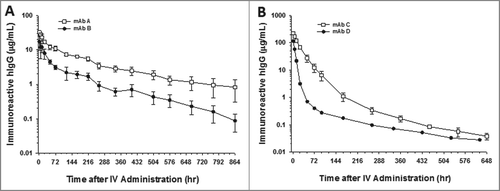
Table 3. Cynomolgus monkey pharmacokinetic parameters of the IgGs
Figure 3. The V308P Fc variant shows no improvements in the cynomolgus monkey pharmacokinetics for a mAb with charge-based NSB, but does improve the clearance of a mAb with no charge-related NSB. Mean pharmacokinetic profiles of (A) mAbs A and A-V308P, which had no charge-associated NSB, and (B) B and B-V308P, which showed binding to heparin in vitro. Individual animal pharmacokinetic profiles of (C) mAbs A and A-V308P and (D) B and B-V308P. Data are the mean ± SD (standard deviation) for 3 animals/time point for all the mAbs. The pharmacokinetics were assessed following a single IV dose of 2 mg/kg for each mAb.
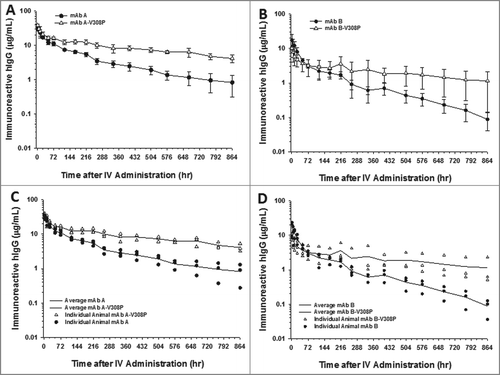
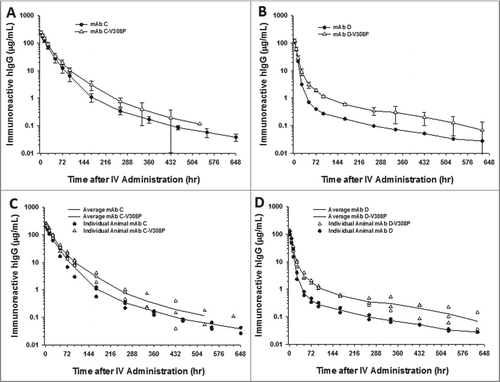
Figure 4. The V308P Fc variant shows no improvements in the cynomolgus monkey pharmacokinetics for mAb with and without charge-based NSB in the presence of a TMD clearance component. Mean pharmacokinetic profiles of (A) mAbs C and C-V308P, which had no charge-associated NSB but bind target antigen, and (B) D and D-V308P, which also bind target antigen, as well as showed binding to heparin in vitro. Individual animal pharmacokinetic profiles of (C) mAbs C and C-V308P and (D) D and D-V308P. Data are the mean ± SD (standard deviation) for 3 animals/time point for all the V308P Fc variant mAbs and 4 animals/time point for mAb C. Data are the mean of 2 animals/time point for mAb D. The pharmacokinetics were assessed following a single IV dose of 10 mg/kg for each mAb.