Figures & data
Figure 1. Immunoprecipitation (IP) of platelet lysates, prepared under native conditions (Panel A) or activated with thrombin (Panel B). Solubilized proteins contained in platelet lysates were subjected to immunoprecipitation with scFv antibodies, immobilized on Protein-A agarose resin. Elution fractions collected were analyzed by SDS-PAGE (10% acrylamide). Panel A: Lane 1: molecular weight marker; Lane 2: scFv alone (no IP); Lane 3: platelet lysate alone (no IP); Lane 4: IP with O52; Lane 5: IP with no scFv (control); Lane 6: IP with R87; Lane 7: IP with N87. It is to be noted that 2 separate gels are presented in Panel A. Panel B: Lane 1: platelet lysate alone (no IP); Lane 2: molecular weight marker; Lane 3: IP with scFv R38; Lane 4: IP with scFv R87.
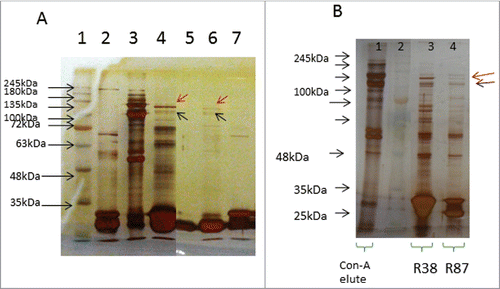
Table 1. Amino acid sequence of CDR regions of heavy chains of the scFv clones
Figure 2. Confocal microscopic images of binding of selected scFv antibodies (50µg/ml), against resting platelets, as well as ADP-activated platelets.
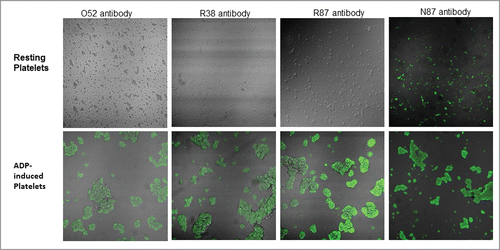
Figure 3. Effect of scFv antibodies on inhibition of ADP-activated platelet aggregation. Platelet-rich plasma was prepared from 4.5 ml of blood sample, collected in 0.5 ml of 3.8% sodium citrate. The platelet count was adjusted to 2 × 108/ml. Aggregation assays were performed at 37°C in siliconized cuvettes at a stirring speed of 900 rpm. 450ul of PRP was taken per tube, and incubated with PAC-1 and scFv antibodies for 20 min. This was followed by addition of 10μM of ADP, and light transmission was recorded till 3 min, and expressed as the percentage of the maximal platelet aggregation in the absence of scFv (PBS). Experiments were conducted using blood drawn from 5 different donors; results were expressed as mean ± SEM.
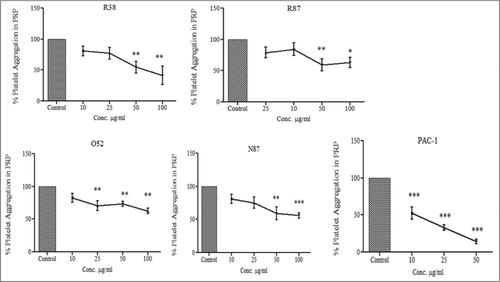
Figure 4. Assessment of binding of scFv antibodies to platelets aggregated by different agonists. Platelets were activated by either ADP (Panel A), or collagen (Panel B), or thrombin (Panel C). In each case mean fluorescence intensity (MFI) of binding to activated platelets vs resting platelets was observed and activation fold (of activated vs resting platelets) was determined. The experiment was repeated in each case with 3 different donors; the data was expressed as mean ± SEM.
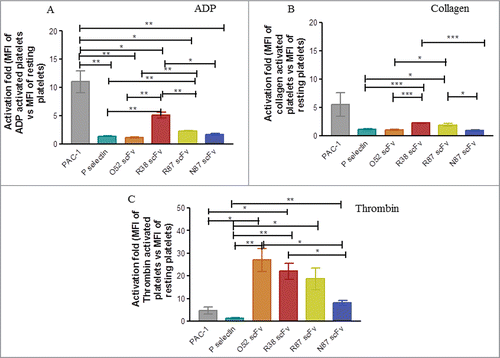
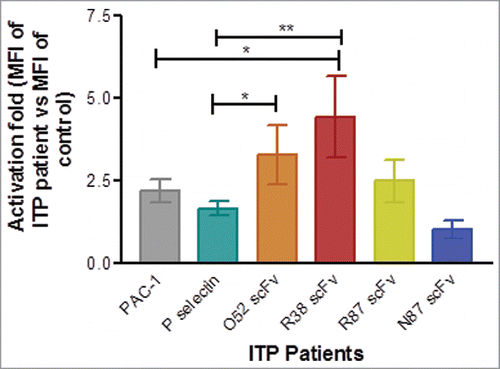
Figure 5. Assessment of platelet activation in healthy controls and ITP patients by flow cytometry. Baseline expression of P-selectin, and binding of PAC-1, and FITC-labeled scFv antibodies was examined. In each case mean fluorescence intensity (MFI) of binding was observed and activation fold was determined in ITP patients in terms of MFI of ITP patients vs. MFI of healthy control for binding of each antibody. Number of patients for PAC-1 and P-selectin = 24. For the scFv antibodies, the number of patients were as follows: scFv R87: n = 14, scFv R38: n = 9, scFv O52: n = 9; scFv N87: n = 14. Data is represented as mean ± SEM.