Figures & data
Figure 1. Characterization of COVA322 (A) Schematic picture of COVA322 showing that the anti-IL-17A Fynomer (orange circle) was genetically fused to the C terminus of the light chain of the anti-TNF antibody adalimumab. (B) COVA322 was expressed transiently in CHO cells and purified using protein A. The resulting protein was >95 % pure and monomeric, as determined by size exclusion chromatography over a period of at least 2 months in a non-optimized phosphate buffered saline buffer. (C) Dual TNF and IL-17A binding of COVA322 is shown using immobilized IL-17A on a BIAcore chip with subsequent injection of COVA322 and TNF (red) or COVA322 only (black) (D) COVA322 and the control anti-IL-17A antibody secukinumab were tested in a cell assay after stimulation with IL-17A and IL-1β. COVA322 and the control antibody specifically inhibited IL-17A with IC50 values of 121 pM and 470 pM, respectively. Mean values of triplicates are shown, error bars represent standard deviations (SD) (E) Gro-α ELISA levels in the supernatants are shown after the stimulation of HT-29 cells with 200 pM TNF and 400 pM IL-17A. After addition of the indicated concentrations of COVA322, a dose-dependent inhibition of IL-17A and TNF can be observed because the Gro-α levels decline with higher concentrations of the inhibitors. As controls, cells were treated with the single cytokines (IL-17A or TNF) or with medium only. Mean values of triplicates are shown, error bars represent standard deviations (SD). (F) COVA322 mediated inhibition of T-cell derived IL-17A induced IL-6 release from HT-1080 cells, comparable to the control anti-IL-17A antibody secukinumab in the presence of excess of anti-TNF antibody adalimumab (Adal.) and anti-IL-1β antibody canakinumab (Cana.). Mean values of quadruplicates are shown, error bars represent standard deviations (SD).
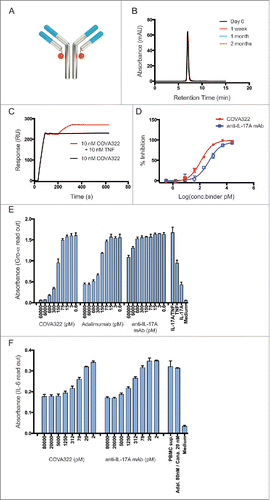
Figure 2. Plasma concentration of COVA322 in cynomolgus monkeys. (A) Bifunctional ELISA was performed to detect intact COVA322. Biotinylated TNF was immobilized in the wells of neutravidin-coated microtiter 96-well plates. Plasma containing COVA322 was added to the wells. For detection, digoxigenin-labeled IL-17A was used, followed by an anti-digoxigenin antibody-HRP conjugate for substrate processing and color development. Monofunctional ELISA was performed to detect specific TNF binding of COVA322, adalimumab or golimumab. Biotinylated TNF was immobilized in the wells of neutravidin-coated microtiter 96-well plates. Plasma containing COVA322 or anti-TNF antibody was added to the wells. TNF binding compound was detected using an anti-human IgG antibody-HRP-conjugate for substrate processing and color development. (B) Plasma concentrations of COVA322 and the anti-TNF antibodies adalimumab and golimumab at different time-points after a single iv injection into cynomolgus monkeys. The COVA322 or anti-TNF antibody concentration in plasma was determined by Bifunctional and Monofunctional ELISA, respectively. Mean plasma concentrations of 3 cynomolgus monkeys are plotted versus time, error bars represent standard deviations (SD). (C) Comparison of the plasma concentrations of COVA322 in cynomolgus monkeys determined by Bifunctional and Monofunctional ELISA. The plasma concentrations obtained from both assays are comparable, indicating that COVA322 is stable in cynomolgus monkeys for at least 220 hours.
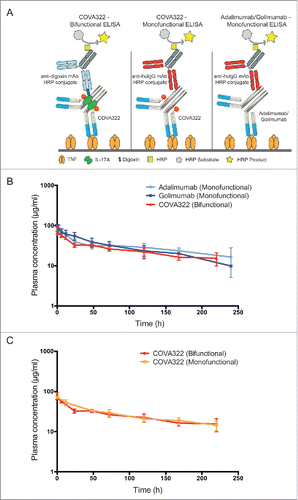
Figure 3. Inhibition of human IL-17A and TNF in vivo. Mice were injected intravenously (iv) with COVA322, anti-IL-17A antibody secukinumab or anti-TNF antibody adalimumab followed by subcutaneous injection of human TNF (A) or human IL-17A (B). Two hours after the administration of the indicated cytokine, blood samples were taken from the mice and KC levels were determined by ELISA. As a control, basal KC levels are shown (mice treated with PBS only (iv), without cytokine stimulation). Mean KC levels of 5 mice per group are shown (± SEM).
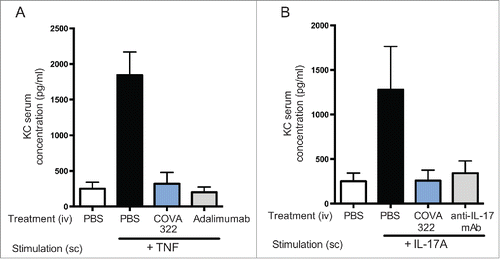
Table 1. Summary of important pharmacokinetic parameters obtained from the non-clinical GLP safety study with cynomolgus monkeys
