Figures & data
Figure 1. AFC mimic drug components. Drug components used in the production of a non-toxic AFC to mimic chemistry and linker species of brentuximab vedotin were based on a (A) dansyl sulfonamide ethyl amine (DSEA) moiety attached to (B) a maleimidocaproyl valine-citrulline linker species (Mal-linker-DSEA). Residual reactive mal-linker-DSEA was quenched with N-acetyl-cysteine following the conjugation step, producing a (C) quenched-linker-fluorophore (NAc-linker-DSEA) adduct species.
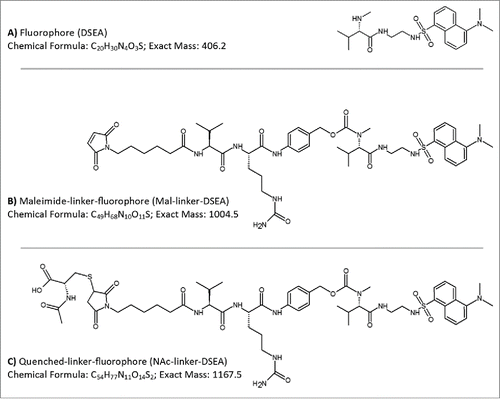
Figure 2. Reference standard evaluation. DSEA, NAc-linker-DSEA, and Mal-linker-DSEA reference standards were separated over a 10 min gradient from 5 % - 50 % (dashed line) with acetonitrile containing 0.1 % FA v/v, as the organic mobile phase using a superficially porous C18 RPLC column. Combined spectrum from SIRs collected using the [M+1H]+1 and [M+2H]+2 charge state for each component using optimized MS settings (see experimental) is shown.
![Figure 2. Reference standard evaluation. DSEA, NAc-linker-DSEA, and Mal-linker-DSEA reference standards were separated over a 10 min gradient from 5 % - 50 % (dashed line) with acetonitrile containing 0.1 % FA v/v, as the organic mobile phase using a superficially porous C18 RPLC column. Combined spectrum from SIRs collected using the [M+1H]+1 and [M+2H]+2 charge state for each component using optimized MS settings (see experimental) is shown.](/cms/asset/787f1888-5071-4f49-b97e-133069283b3d/kmab_a_1116659_f0002_b.gif)
Figure 3. Assay dynamic range. Analysis of reference standards were performed in triplicate. Calibration plots of the reference standards were generated using peak area from SIRs for the most abundant [M+2H]+2 charge state and fitted with an ordinary linear regression model. Using ICH guidelines the MS quadrupole dynamic range was determined to be 1.35 pg – 688.5 pg for the mal-linker-DSEA and 1.65 pg – 854.5 pg for the NAc-Linker-DSEA reference standards.
![Figure 3. Assay dynamic range. Analysis of reference standards were performed in triplicate. Calibration plots of the reference standards were generated using peak area from SIRs for the most abundant [M+2H]+2 charge state and fitted with an ordinary linear regression model. Using ICH guidelines the MS quadrupole dynamic range was determined to be 1.35 pg – 688.5 pg for the mal-linker-DSEA and 1.65 pg – 854.5 pg for the NAc-Linker-DSEA reference standards.](/cms/asset/9a497138-43b2-47c9-b58c-fe2787ad2ca7/kmab_a_1116659_f0003_b.gif)
Table 1. Assay suitability. Analyses of standards were performed in triplicate and evaluated using ICH guidelines for precision (< 20 % R.SD at the LOQ, otherwise <15 %) and accuracy (< 20 % relative error (R.E.) at the LOQ, otherwise <15 %). The dynamic range was extended 2 orders of magnitude using the quadrupole MS detector in a serial configuration with the LC-TUV optical detector.
Figure 4. Instrument configuration schematic. (A) A column manager housing 2 6-port 2-position valves was configured as illustrated by the schematic to facilitate transfer of retained drug species between the SPE (1st) and RPLC (2nd) dimensions. Valve position is denoted numerically as position 1 and 2. Abbreviations are defined as QSM: quaternary solvent manager, AS: auto sampler, TUV: tunable ultraviolet detector, BSM: binary solvent manager, MS: mass spectrometer, ACD: at-column-dilution. (B) Extracted drug species were transferred in a 5.50 min elution window using at-column-dilution with both valves in position 2 to refocus eluting drug species at the head of the analytical column. The transfer was bracketed with a 0.6 second interval in position 2,1 to purge the fluidic path.
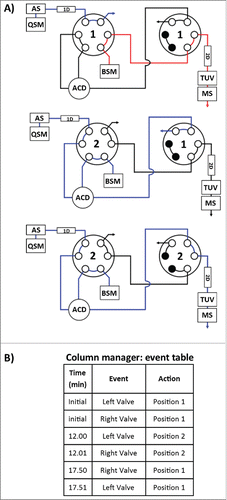
Figure 5. Method evaluation of SPE with spiked sample. Mal-linker-DSEA, and NAc-linker-DSEA was spiked into a dilute trastuzumab sample for SPE optimization. Optimal SPE loading conditions for the extraction of mal-linker-DSEA and NAc-linker-DSEA components from the spiked AFC sample were determined to be 18% acetonitrile containing 2% FA v/v. A step gradient to 36% acetonitrile containing 2% FA v/v was determined to be optimal conditions to elute bound drug components in a narrow peak centered around 13.5 min.
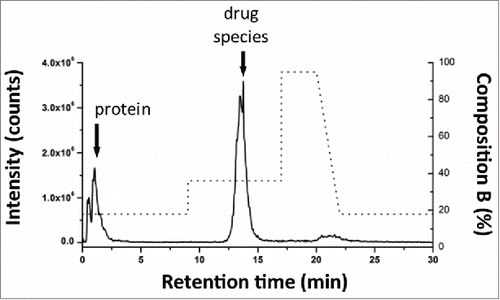
Figure 6. Evaluation of 2DLC configuration. NAc-linker-DSEA, spiked into a dilute trastuzumab sample, was successfully transferred from (A) the SPE column (1st dimension) to the (B) RP column (2nd dimension) using the 2DLC configuration illustrated in A as proof-of-principle.
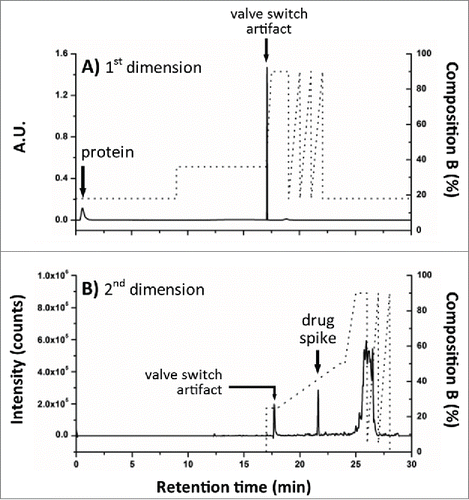
Table 2. 2DLC recovery evaluation. Nac-linker-DSEA reference standard was prepared at 4 concentrations throughout the experimentally determined dynamic range and evaluate for recovery efficiency using the 2DLC configuration illustrated in A. Identical separations were performed in a 1DLC configuration using the same system with a union in lieu of the SPE column (1st dimension) as a reference. Comparison of peak area across triplicate injections of the NAc-linker-DSEA reference standard indicate sample recovery was equivalent between both 1DLC and 2DLC configurations.
Table 3. AFC sample results. NAc-linker-DSEA and mal-linker-DSEA were detected at levels below UV detection thresholds in the AFC sample at concentrations of 7.19 ng/mL and 3.82 ng/mL, respectively.
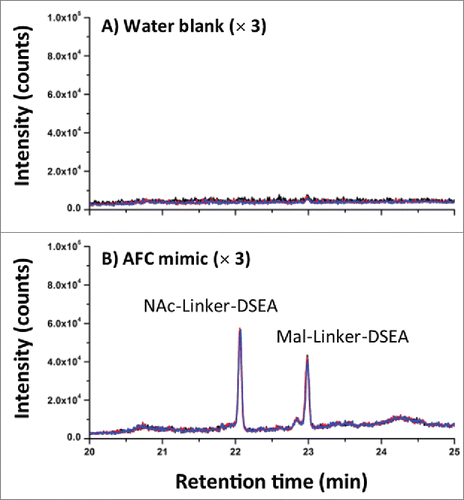
Figure 7. AFC sample chromatography. (A) Using the optimized 2DLC method as shown in , a water blank was performed prior to each DSEA sample injection to monitor carry-over between DSEA sample injections. Overlay chromatograms of the 3 water blanks indicate no observable carry over of NAc-linker-DSEA and negligible carry over of mal-linker-DSEA (< 5% by area) between runs. (B) NAc-linker-DSEA and Mal-linker-DSEA drug components were detected in a 10 uL injection (19.4 ug) of neat AFC sample. Overlays of the 3 runs indicate a high degree of assay precision.