Figures & data
Figure 1. 9A2 inhibits IL-3/GM-CSF/IL-5 induced TF-1 proliferation and STAT5 phosphorylation, and binds specifically to the human βc receptor. (A) TF-1 cells were starved of growth factor for 18 h and then treated with test antibodies BION-1 (○) and 9A2 (•) for 30 min prior to the addition of cytokines (IL-3, GM-CSF, IL-5) for 72 hr. Proliferation was assayed by [H3]-thymidine incorporation and the IC50 values plotted. TF-1-bla cells were starved of growth factor for 18 hours and then treated with 9A2 for 30 minutes prior to the addition of cytokines. For pStat5 assays TF-1-bla cells were incubated with (B) IL-3 (10 ng/ml) or (C) GM-CSF 3 ng/ml) at 37°C for 5 h and FRET B/G substrate was added for 2.5 hours prior to reading the output on an Envision plate reader. Histograms show mean and standard error of technical replicates. Representative experiments are shown. (D) FreeStyle™ 293-F cells were transiently transfected with cDNAs encoding the βcR, the GMRα, the IL-3Rα and the IL5Rα and analyzed by flow cytometry. Control antibodies to the human βcR, GMRα, the IL-3Rα or the IL-5Rα confirmed expression of these proteins in the transfected cells.
![Figure 1. 9A2 inhibits IL-3/GM-CSF/IL-5 induced TF-1 proliferation and STAT5 phosphorylation, and binds specifically to the human βc receptor. (A) TF-1 cells were starved of growth factor for 18 h and then treated with test antibodies BION-1 (○) and 9A2 (•) for 30 min prior to the addition of cytokines (IL-3, GM-CSF, IL-5) for 72 hr. Proliferation was assayed by [H3]-thymidine incorporation and the IC50 values plotted. TF-1-bla cells were starved of growth factor for 18 hours and then treated with 9A2 for 30 minutes prior to the addition of cytokines. For pStat5 assays TF-1-bla cells were incubated with (B) IL-3 (10 ng/ml) or (C) GM-CSF 3 ng/ml) at 37°C for 5 h and FRET B/G substrate was added for 2.5 hours prior to reading the output on an Envision plate reader. Histograms show mean and standard error of technical replicates. Representative experiments are shown. (D) FreeStyle™ 293-F cells were transiently transfected with cDNAs encoding the βcR, the GMRα, the IL-3Rα and the IL5Rα and analyzed by flow cytometry. Control antibodies to the human βcR, GMRα, the IL-3Rα or the IL-5Rα confirmed expression of these proteins in the transfected cells.](/cms/asset/f6ac97fa-58c0-454b-943b-18d28d890f66/kmab_a_1119352_f0001_b.gif)
Figure 2. Affinity maturation of 9A2 results in Fabs with improvements in dissociation rate (kd) and affinity (KD). Binding of (A) 9A2 Fab, (B) 9A2-VR24 Fab and (C) 9A2-V24.29/CSL311 Fab to immobilized βc was determined by surface plasmon resonance. Sensorgram (colored) and fitted curves (black) for each concentration are shown. The kinetic constants can be found in Tables S1 and S2.
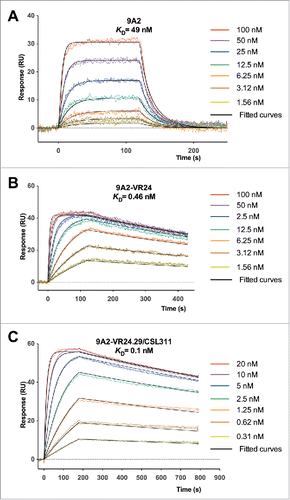
Figure 3. Affinity maturation of 9A2 results in mAbs with improved potency against IL-3, GM-CSF and IL-5. (A) For the first round of affinity maturation 6 different CDR libraries were subjected to 5 rounds of selection in solution with immobilized βc-Fc protein, where the concentration was sequentially reduced with each round. Affinity-matured phage were isolated and unique variants were re-formatted into full length IgG4 antibodies. Variants 9A2-VR24 (▾) and 9A2-VR39 (♦) were selected for the second round of affinity maturation. (B) Affinity-matured phage were isolated and unique variants re-formatted into full length IgG4 antibodies. All affinity-matured variants from each library were tested for potency against GM-CSF in TF-1 proliferation assays and individual IC50 values plotted. 9A2-VR-24.29/CSL311 (▴). TF-1 cells were starved of growth factor for 18 h and then treated with test antibodies 9A2 (○), 9A2-VR24 (□) and 9A2-VR24.29/CSL311 (Δ)) for 30 min prior to the addition of cytokines. (C) IL-3. (D) GM-CSF. (E) IL-5. Cells were incubated at 37°C for 72 h and pulsed with 3[H]-thymidine for the final 6 h then harvested onto glass filters and read on a β-counter. Histograms show mean and standard error of technical replicates. Experiments were repeated at least 3 times. Representative experiments are shown.
![Figure 3. Affinity maturation of 9A2 results in mAbs with improved potency against IL-3, GM-CSF and IL-5. (A) For the first round of affinity maturation 6 different CDR libraries were subjected to 5 rounds of selection in solution with immobilized βc-Fc protein, where the concentration was sequentially reduced with each round. Affinity-matured phage were isolated and unique variants were re-formatted into full length IgG4 antibodies. Variants 9A2-VR24 (▾) and 9A2-VR39 (♦) were selected for the second round of affinity maturation. (B) Affinity-matured phage were isolated and unique variants re-formatted into full length IgG4 antibodies. All affinity-matured variants from each library were tested for potency against GM-CSF in TF-1 proliferation assays and individual IC50 values plotted. 9A2-VR-24.29/CSL311 (▴). TF-1 cells were starved of growth factor for 18 h and then treated with test antibodies 9A2 (○), 9A2-VR24 (□) and 9A2-VR24.29/CSL311 (Δ)) for 30 min prior to the addition of cytokines. (C) IL-3. (D) GM-CSF. (E) IL-5. Cells were incubated at 37°C for 72 h and pulsed with 3[H]-thymidine for the final 6 h then harvested onto glass filters and read on a β-counter. Histograms show mean and standard error of technical replicates. Experiments were repeated at least 3 times. Representative experiments are shown.](/cms/asset/0d3cd281-b16b-4945-8b9c-c0564da47aef/kmab_a_1119352_f0003_b.gif)
Figure 4. CSL311 competes with IL-3, GM-CSF, and IL-5 for binding to primary human myeloid cells. Purified human eosinophils (A,C) or neutrophils (B) were pre-incubated with CSL311 (•) or a human IgG4 control antibody (○). Cells were then equilibrated with (A) 340 pM radio-iodinated IL-3, (B) 40 pM radio-iodinated GM-CSF (C) 200 pM radio-iodinated IL-5. Each point is the mean of duplicate determinations of cell-bound radioiodinated cytokine and error bars represent the standard deviation. Data from representative experiments is shown, n=2. (D) TF-1 cells were pre-incubated with IL-3 (▴), GM-CSF (▪) or IL-5 (•) then cells were equilibrated with 85 pM radio-iodinated CSL311. Each point is the mean of duplicate determinations of cell-bound radio-iodinated CSL311 and error bars represent the standard deviation. Data from a representative experiment is shown, n = 3.
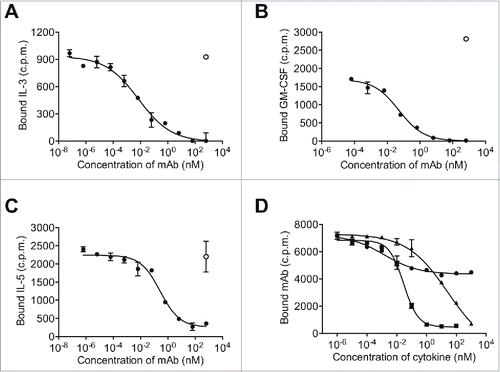
Figure 5. The Site 2 Interaction interface between CSL311 and the βc receptor. (A) Superimposition of the βc/CSL311 Fab complex on the GM-CSF receptor ternary structure, showing the overlap of the interaction interface between CSL311 Fab (heavy chain shown in orange and light chain in yellow) and GM-CSF (gray). The βc dimer from the GM-CSF ternary structure is colored in green and purple. (B) A surface representation of the key residues on the βc dimer that interact with CSL311 is shown. Individual residues are colored to indicate the effect of specific alanine substitution mutations on CSL311 binding affinity: mutations that lead to no binding or negligible binding are colored in red, mutations that reduce binding to the 10−5 to 10−7 M range are shown in yellow and mutations that improve binding are shown in blue. The detailed interactions involving CDRs H1-H3 and CDR L1 and L3 are shown in the adjoining zoom-in panels. Polar interactions are shown as black broken lines and key van der Waals interactions are shown in yellow. All figures were made using PyMOL.
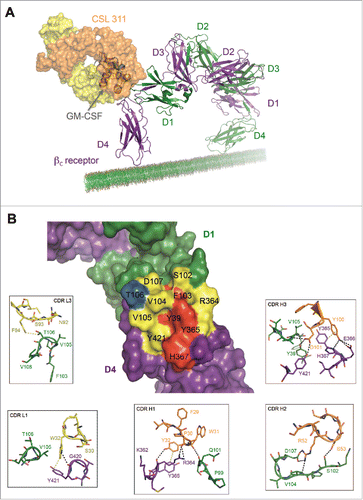
Figure 6. CSL311 inhibits the survival of primary human eosinophils cells following stimulation with βc-signaling cytokines. Eosinophils were pre-treated with CSL311 (▴), anti-IL-5Rα (□, 66.7 nM), anti-IL-3Rα (▵, 66.7 nM), anti-GMRα (◊, 66.7 nM), or anti-IL-5Rα/anti-IL-3Rα/anti-GMRα, (•, 66.7 nM each mAb) before treatment with IL-3/GM-CSF/IL-5, for 72h and cell survival determined. No antibody added (▪). Data from representative experiments are shown, n = 5. All values expressed as mean +SEM. Experiments were repeated at least 4 times with representatives shown.
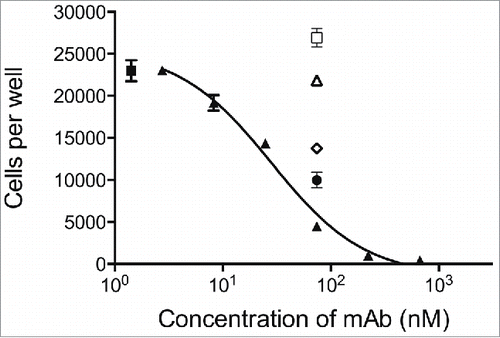
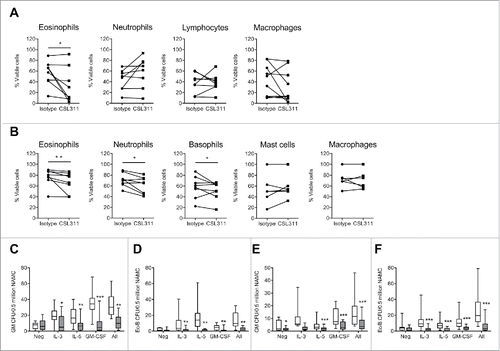
Figure 7. CSL311 inhibits survival of cells isolated from human inflammatory airway disease tissue. Sputum cells from samples induced at baseline and 24 h after inhaled allergen challenge from allergic asthmatic subjects were incubated with (A) CSL311 (100 µg/ml) or an isotype control mAb (100 µg/ml) at 37°C for 24 h or (B) incubated with CSL311 (100 µg/ml) or an isotype control mAb (100 µg/ml) at 37°C for 24 h in the presence of 1 ng/ml each IL-3, GM-CSF and IL-5. Cells were analyzed for viability by flow cytometry. Data are expressed as percent cell viability and compared to the isotype control for each donor. Samples where the pre-incubation viability was<10% were excluded from the analysis. Non-adherent mononuclear cells (NAMC) from (C-D) bone marrow and (E-F) blood samples collected at baseline and 24 h after inhaled allergen challenge from allergic asthmatic subjects were incubated with CSL311 (100 µg/ml, filled bars) or an isotype control mAb (100 µg/ml, open bars) at 37°C for 24 h in the presence of diluent (neg) or 1 ng/ml each IL-3, GM-CSF and IL-5, or cytokines combined (All) and (C,E) GM CFU and (D,F) Eo/Baso CFU were enumerated. Data expressed as median ± range and 95% confidence intervals. A Wilcoxon matched pairs signed rank 2 tailed t-test was used to determine statistical significance: ns, not significant p > 0.05; * p < 0.05; ** p < 0.01; *** p < 0.005.