Figures & data
Figure 1. Comparison of the frequency of donor DRB (a) and DQB (b) allotypes expressed in the European population against the whole study donor cohort (n = 68), and donors expressing the homozygous G1m3 allotype (n = 37) and donors expressing the homozygous G1m1,17 allotype (n = 31)
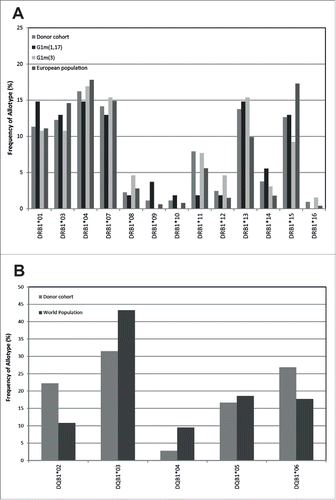
Figure 2. Healthy donor T-cell proliferation to IgG1 G1m3-trastuzumab and G1m1,17-trastuzumab. T-cell proliferation was measured from bulk cultures of PBMC on days 5, 6, 7 and 8. Figure shows the maximum stimulation index over the 4 day period. The threshold for positive T-cell proliferation (SI ≥ 1.90, p < 0.05) is indicated by red dotted line. Borderline responses (SI = 1.90-1.95, p < 0.05) are indicated (*). Donors expressing homozygous G1m3 (a and c) and G1m1,17 (b and d) were tested against G1m3-trastuzumab (a and b) and G1m1,17-trastuzumab (c and d)
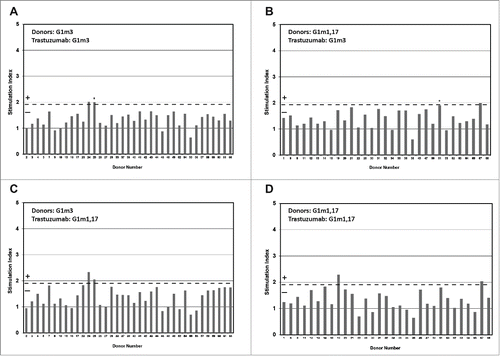
Figure 3. Healthy donor IL-2 ELISpot responses to IgG1 G1m3-trastuzumab and G1m1,17-trastuzumab. Replicate cultures were incubated in the presence of test samples for a total of 8 days (in parallel to the proliferation assays) prior to detection of secreted IL-2. The threshold for positive IL-2 ELISpot responses (SI ≥ 1.90, p < 0.05) is indicated by the dotted line. Borderline responses (SI = 1.90-1.95, p < 0.05) are indicated (*). (a) homozygous G1m3 donors and (b) homozygous G1m1,17 donors. No ELISpot data was obtained from donors 32 and 49.
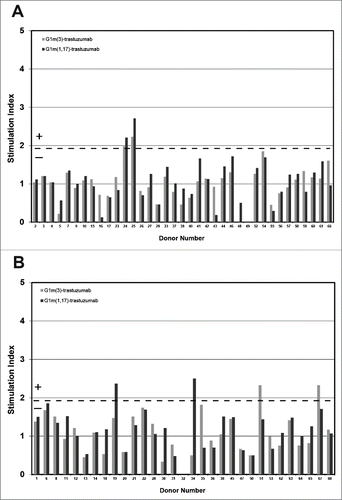
Table 1. Summary of human IGγ1 (G1m) allotypes and their associated genotypes (From: http://www.imgt.org).
Table 2. Summary of G1m3-trastuzumab and G1m1,17-trastuzumab specific T-cell proliferation and IL-2 ELISpot responses in the homozygous G1m3 and G1m1,17 donor cohorts. Frequency (expressed as a percentage of the donor cohort) of positive proliferation responses (SI ≥ 1.9, significant p < 0.05) and IL-2 responses (SI ≥ 1.9, significant p < 0.05).
Figure 4. Healthy donor proliferation responses to peptides containing: G1m1 (peptides 1 and 2), nG1m1 (peptides 4 and 5), G1m3 (peptide 6) and G1m17 (peptide 7). Replicate cultures were incubated in the presence of peptides for a total of 7 days prior to assay for proliferation with groups of: (a) homozygous G1m3 donors, (b) homozygous G1m1,17 donors and (c) heterozygous donors. The background threshold for positive (SI ≥ 1.90, p < 0.05) proliferation is indicated by the dotted line (average responses +2 SD).
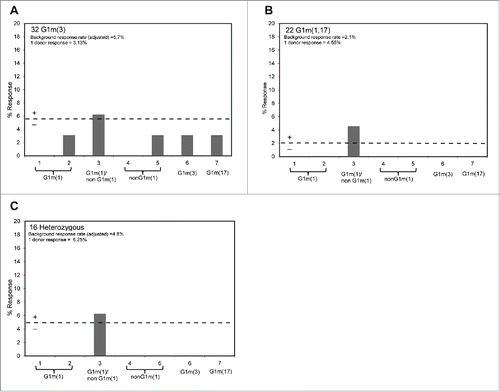
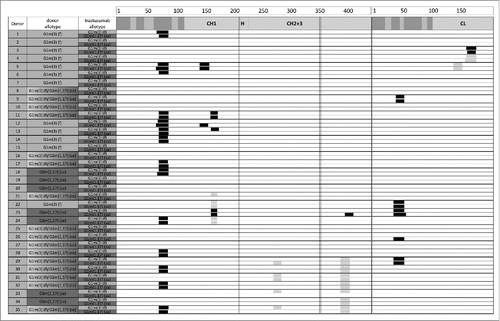
Figure 5. HLA-DR–associated peptides produced by 5 × 106 dendritic cells from 35 different donors exposed separately to G1m1,17 and G1m3 allotypes of trastuzumab. HLA-DR–associated peptides can originate from various regions of a protein typically occurring as multiple length variants that share the same HLA-DR binding core and form a “cluster." Clusters are indicated as black boxes, CDRs are indicated as shaded areas along the sequence of heavy chain (left) and light chain (right). Allotypic variations are indicated as vertical lines in the heavy chain. Clusters that were also detected in the media control samples are indicated as gray boxes. The detection of these peptides in the control samples can be explained by the use of human serum containing human IgG in the PBMC freezing media.