Figures & data
Figure 1. (A) Chemical structure of antibody-SMCC-DM1 and antibody-SPDB-DM4 conjugates. (B) Scheme of antibody conjugation described in this work.
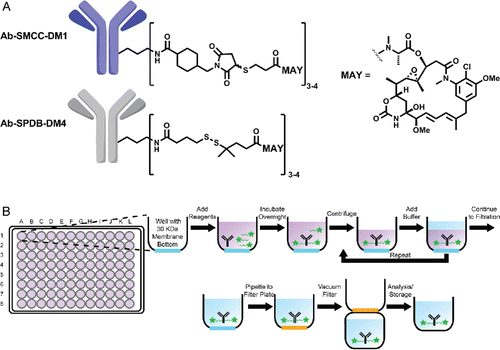
Figure 2. Initial qualification of conjugation using the microscale conjugation platform. Each of 3 antibodies was conjugated at microscale (3 replicates each, 100 µg per replicate) with SMCC-DM1 and SPDB-DM4 at pH 8, and with SMCC-DM1 at pH 6. (A) Yield as determined by volume measurement and concentration from SE-UPLC. (B) % Monomer and (C) DAR, each determined by SE-UPLC. Each bar is an average + SD of 3 replicates.
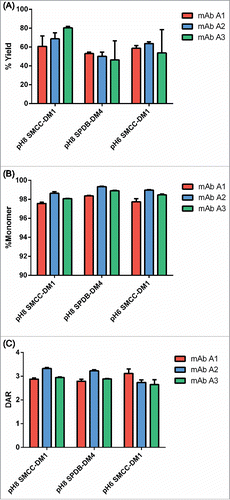
Figure 3. Example titrations of different human IgG1s with SMCC-DM1 using antibody stocks of different concentrations. Each point is the average of 2 replicate purified conjugation reactions. Each reaction contained 100 µL of antibody stock at the indicated concentration in a 200 µL reaction volume. Antibodies: (A) mAb A2; (B) mAb A3; (C) mAb A4.
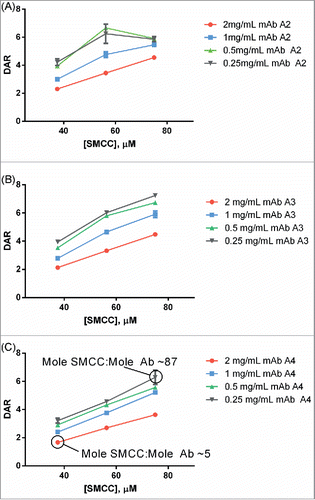
Figure 4. (A) Dependence of DAR on antibody concentration at pH 8 and pH 6. At pH 8, as antibody concentration increases, the DAR steeply decreases. At pH 6, the DAR is insensitive to antibody concentration changes. For pH 8, the concentration of SMCC-DM1 is 38 µM for all points; for pH 6, the concentration of SMCC-DM1 is 150 µM for all points. Each point is the average of 2 microscale reactions. (B) Reaction progress at pH 6 was measured by setting up a reaction in an autosampler vial and measuring the DAR of the crude reaction product by SE-UPLC at the indicated times.
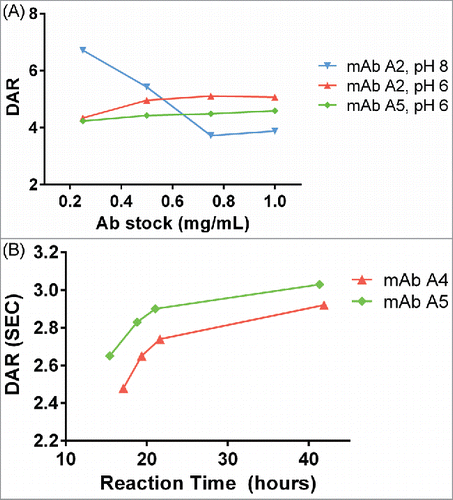
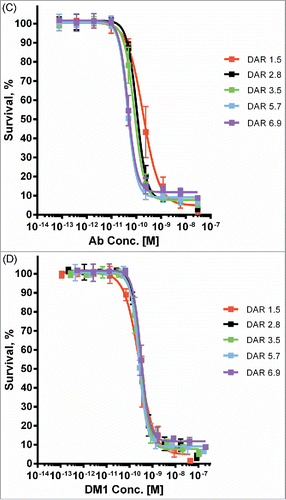
Figure 5. Cytotoxic potency of maytansinoid ADCs produced by microscale methods. (A) Microscale anti-CD33 conjugates produced at both pH 8 and pH 6 were compared with conjugate produced using standard methods were assayed for cytotoxic potency against MOLM-13 cells, both alone (solid curves) and in the presence of saturating unconjugated antibody (1 μM) to block specific uptake (dashed curves). (B) Cytotoxicity assay of identical mAb A2-SMCC-DM1 ADCs which were purified by the indicated number of wash steps. The cell line used (JeKo-1) does not express the antigen recognized by mAb 2, hence the cytotoxicity observed is due only to nonspecific uptake or cytotoxic impurities. Each wash consisted of the addition of fresh buffer and ultrafiltration. (C) mAb A6-SMCC-DM1 conjugates were generated with a range of DAR using microscale conjugation at pH 8. These conjugates were then assayed for cytotoxic potency on a high antigen (EGFR) density cell line (MDA-MB-483). The kill curves are plotted with antibody concentration as the X-axis. (D) The same data as in part C, but plotted with DM1 concentration as the X-axis.
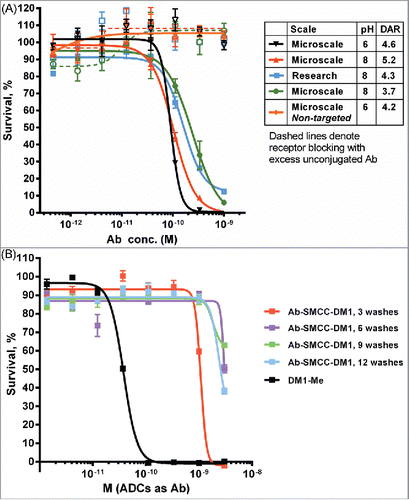
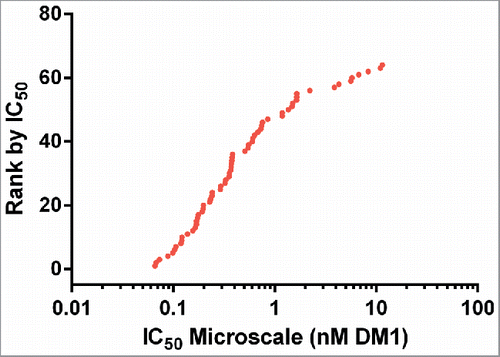
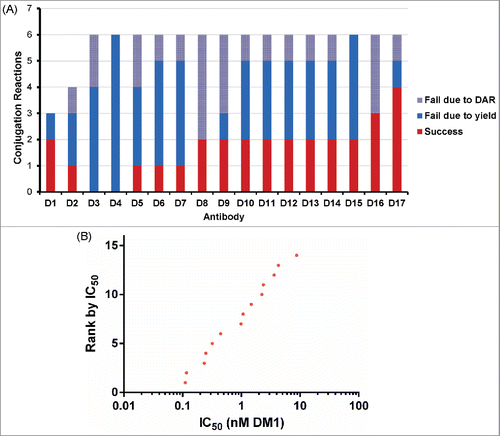
Figure 6. Agreement between IC50 values determined for research and microscale SPDB-DM4 ADCs targeting Antigen B. For each antibody, conjugates made by both methods were simultaneously assayed for cytotoxicity on cells expressing Antigen B. (A) Agreement between research and microscale ADCs depicted in a Bland-Altman plot comparing IC50 values in log space. The mean of the differences between assays = 0.41, so the bias is 100.41, corresponding to a 2.6-fold mean difference between microscale and research scale, with microscale appearing more potent. The standard deviation of the differences between assays = 0.38, so the 95% confidence interval of a pair of microscale and research scale IC50s = 100.76 = 5.6-fold above and below the mean. (B) ADCs targeting Antigen B were ranked based on cytotoxic IC50 using research scale and microscale conjugates, and the rank order is compared.
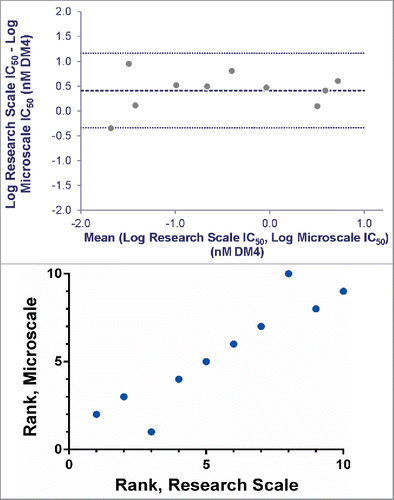
Table 1 Summary of conjugation reactions for 85 antibodies against Antigen C.