Figures & data
Figure 1. Mean (± SD) serum concentration−time profiles following a single IV administration of 1, 10, and 30 mg·kg−1 PRO304397 to BALB/c mice. N = 3 mice per time point per group. The minimum quantifiable concentration (MQC) in the PRO304397 concentration ELISA was 0.0078 µg·mL−1. Values below MQC were interpreted as missing for summary statistics.
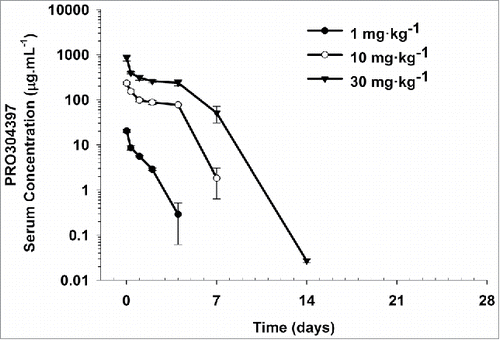
Figure 2. Saturation of PDL1 on CD8+ (A) and CD4+ (B) T lymphocytes following single IV administration of 1, 10, and 30 mg·kg−1 PRO304397 to BALB/c mice. N = 3 mice per group/per time point, where 3 mice were sacrificed at each time point per group. Saturation of PDL1 was assessed via flow cytometry by measuring the amount of free membrane PDL1 present on the cell surface of peripheral blood CD8 + and CD4 + T lymphocytes. Biotin-conjugated PRO304397 was used to stain peripheral blood mononuclear cells and detected with streptavidin-phycoerythrin (SA-PE). Values were normalized against an appropriate isotype control. Mean (+/−SD) fluorescence values for each group are represented as molecules of equivalent fluorescence (MOEF).
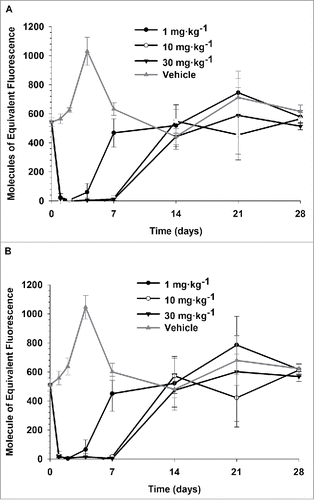
Figure 3. Mean (± SD) serum concentration-time profiles following a single IV administration of 0.5, 5, and 20 mg·kg−1 MPDL3280A to cynomolgus monkeys. N = 4 animals per group. The minimum quantifiable concentration (MQC) in the MPDL3280A concentration ELISA was 0.075 μg·mL−1. Values below MQC were interpreted as missing for summary statistics.
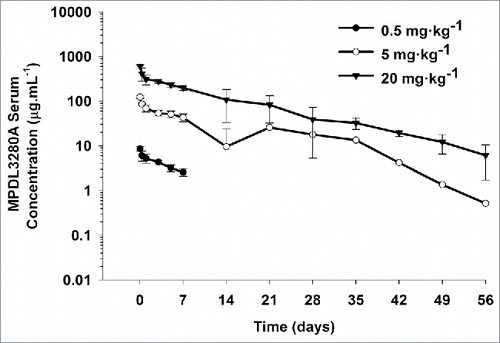
Figure 4. Plasma and tissue radioactive level following a single IV bolus administration of 125I- PRO304397 along with 0–40 mg·kg−1unlabeled antibody to MC38 tumor-bearing mice. (A) Plasma radioactivity levels at 24 and 168 h post-dose. The data are presented as average percent injected dose per milliliter of plasma (%ID·mL−1). (B) The tissue radioactivity levels at 24 h post-dose. The data are presented as average percent injected dose per gram of tissue (%ID·g−1). (C) Tumor interstitial to plasma ratio at 24 and 168 h post-dose. For all figures, the error bars are the standard deviation of 3 measurements.
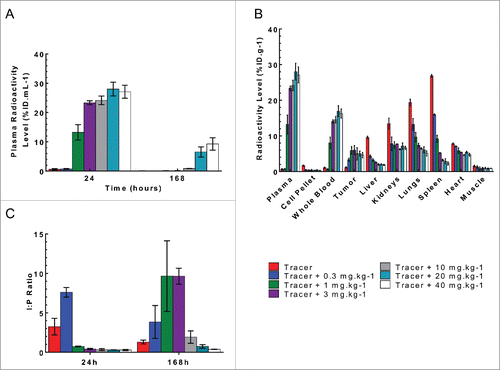
Figure 5. Plasma and tissue radioactive level following a single IV bolus administration of 125I- PRO304397 along with 0–40 mg·kg−1unlabeled antibody to Cloudman tumor-bearing mice. (A) Plasma radioactivity levels at 24, 120, and 168 h post-dose. The data are presented as individual animal percent injected dose per milliliter of plasma (%ID·mL−1) with a line passing through the average. (B) The tissue radioactivity levels at 24 h post-dose. The data are presented as average percent injected dose per gram of tissue (%ID·g−1). (C) Tumor interstitial to plasma ratio at 24, 120, and 168 h post-dose. For all figures, the error bars are the standard deviation of 3 measurements.
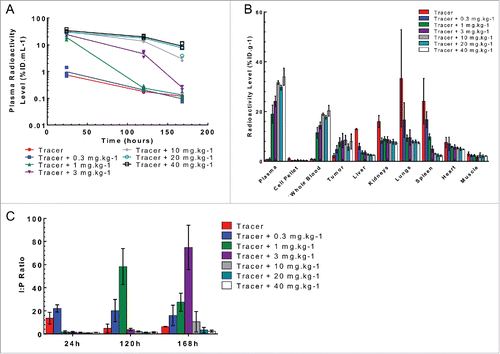
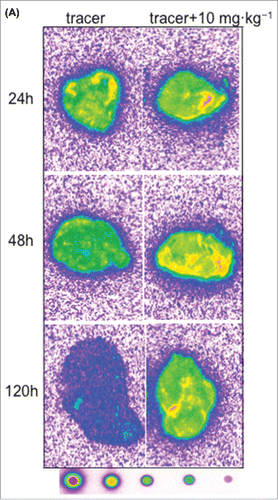
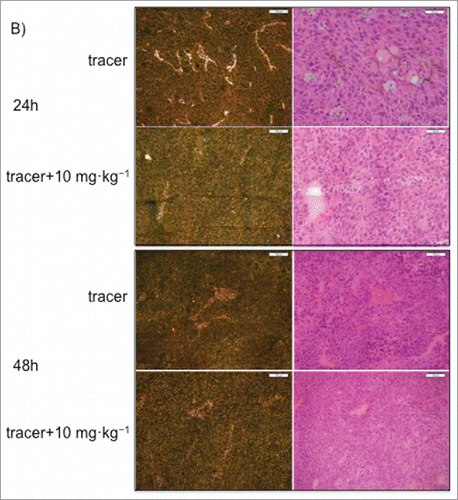
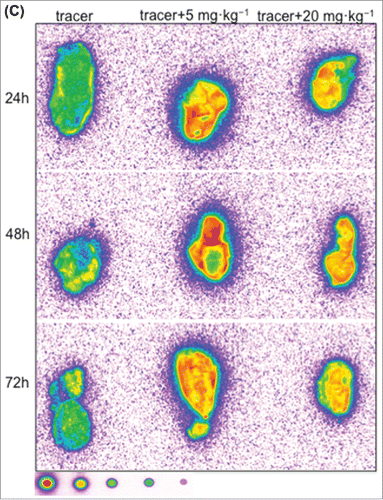
Figure 6. Representative autoradiography images of sagittal tumor sections in tumor-bearing mice following intravenous administration of 125I- PRO304397 tracer or 125I- PRO304397 tracer + unlabeled PRO304397. (A) Autoradiography sections of MC38 tumors at 24, 48, and 120 h after administration of 125I-PRO304397 tracer alone or 125I- PRO304397 tracer + 10 mg·kg−1 unlabeled PRO304397, respectively. A heat map (below the tumor images) shows the range of radioactivity from red being the highest to purple the lowest. (B) Representative sagittal sections of MC38 tumors showing cell distribution of 125I- PRO304397-associated signal by MAR (left) and H&E (right), respectively at 24 and 48 h following 125I- PRO304397 tracer alone or 125I- PRO304397 tracer + 10 mg·kg−1unlabeled PRO304397. (C) Autoradiography sections of Cloudman tumors at 24, 48, and 72 h after administration of 125I- PRO304397 tracer alone or 125I-PRO304397 tracer+ unlabeled PRO304397 at 5 and 10 mg·kg−1, respectively. Heat map depicts pseudocolor intensities as described above.