Figures & data
Figure 1. (A) Schematic representation of the cross-arm binding efficiency (χ), (B) the ligand-binding model structure for JNJ-61186372, an EGFR x c-Met bispecific antibody.
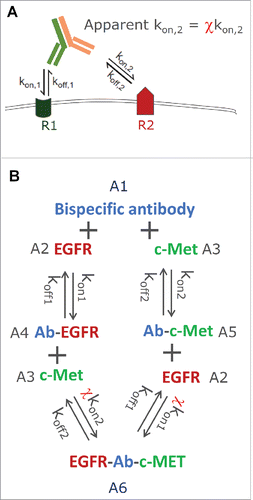
Figure 2. Simultaneous fitting of the EGFR and c-Met monovalent parent (gp120 x EGFR Ab and gp120 x c-Met Ab) binding data to 8 NSCLC cell lines, model fitting (line) vs. observed data (symbols).
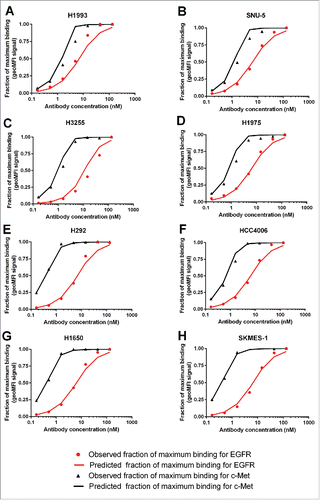
Table 1. EGFR and c-Met receptor densities and model derived cell densities of the 8 NSCLC cell lines used in flow cytometry staining.
Table 2. Model input parameter and model-estimated parameter values.
Figure 3. Estimation of the χ value of JNJ-61186372 by simultaneously fitting the ligand-binding model to JNJ-61186372 binding data to 3 NSCLC cell lines with similar EGFR and c-Met receptor densities.
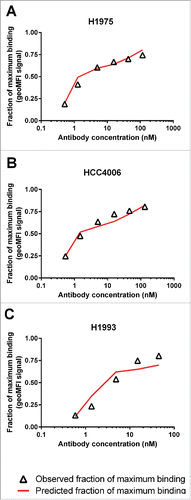
Figure 4. (A) Model-predicted binding of JNJ-61186372 to EGFR and c-Met, respectively, on H1993 and H292; (B) Correlation between the model-predicted EC50 for EGFR and c-Met binding and the observed IC50 values for EGFR and c-Met phosphorylation inhibition in H1993 and H292 cell lines.
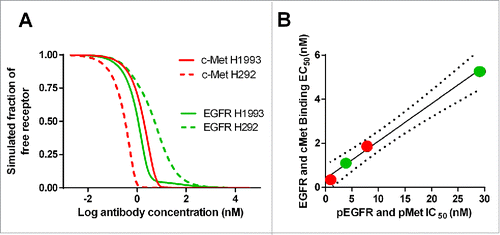
Figure 5. Simulated profiles of the free fraction of EGFR and c-Met in the H1975/HGF mouse xenograft model for JNJ-61186372 and the combination of both monovalent parent antibodies (gp120 x EGFR Ab + B21M x c-Met Ab).
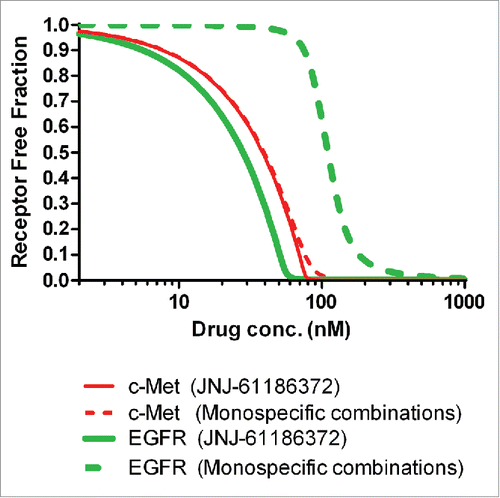
Figure 6. Tumor growth inhibition profiles by JNJ-61186372 (10mg/kg) and the combination therapy of both monovalent antibodies (10mg/kg+10mg/kg) in a H1975/HGF mouse xenograft model (Dosing = BIW × 3 weeks) *P < 0.05, #P < 0.1 based on a 2-tailed t-test for each studied day.
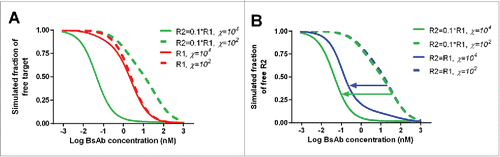
Figure 7. Model simulation to show the effect of χ on the binding of a bispecific antibody (BsAb) to receptors (A) χ has more significant effect on the binding of a BsAb to the second receptor (R2), than the anchor receptor (R1, which is assumed to have 10-fold higher abundance and 10-fold higher affinity); (B) The effect of χ on R2 binding is more apparent when the R2 baseline density is lower (R1 is assumed to have 10-fold higher affinity).