Figures & data
Table 1. Dissociation constants (KD) for binding of the novel Fc variants to human and cynomolgus monkey FcRn at pH 6.0.
Figure 1. Dot plots and statistical significance in RF binding assay for variants with (a) YTE, (b) N434H, and (c) LS. Each individual response of healthy donors and RA patients is shown with median and interquartile range (+/− IQR).
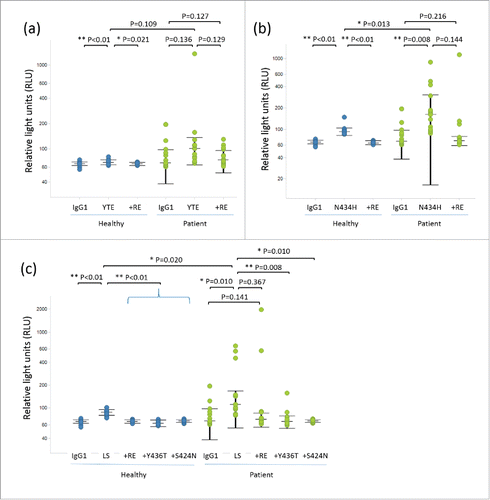
Figure 2. Complex structures of (a) CH2 and CH3 domains of Fc (green) with RF heavy chain (light blue) and light chain (purple), and of (b) CH2 and CH3 domains of Fc (green) with FcRn α chain (yellow) and β-2-microgloblin (red). Enlarged figures of (a) and (b) are shown as (c) and (d), respectively, and the positions mutated to enhance FcRn binding (252, 254, 256, 428 and 434) are shown as orange sticks.
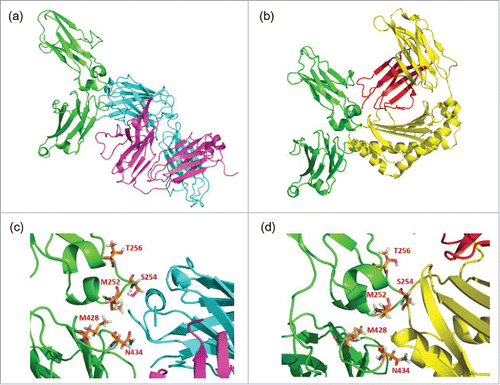
Figure 3. Enlarged complex structures of (a) Fc and RF, (b) Fc and FcRn, and (c) Fc and Protein A. In each figure, the positions mutated to enhance FcRn binding (252, 254, 256, 428, and 434) are shown as orange sticks, and those to reduce RF binding (424, 436, 438, and 440) are shown as blue sticks. The colors of ribbons are the same as in . The blue sticks interact with the light chain of RF but not with FcRn or protein A. Surface figures of each complex structure are shown in (d), (e), and (f). The colors of the molecular surface are the same in as the ribbon structures. The blue regions, which indicate the positions mutated to reduce RF binding, are exposed to the molecular surface in (e) and (f), but not in (d).
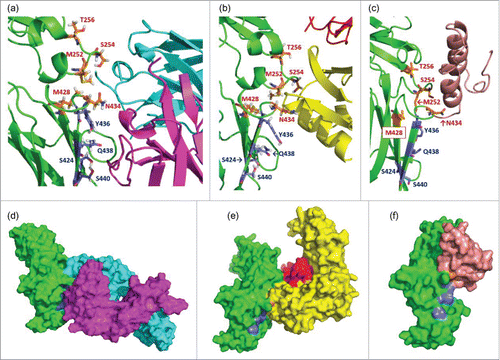
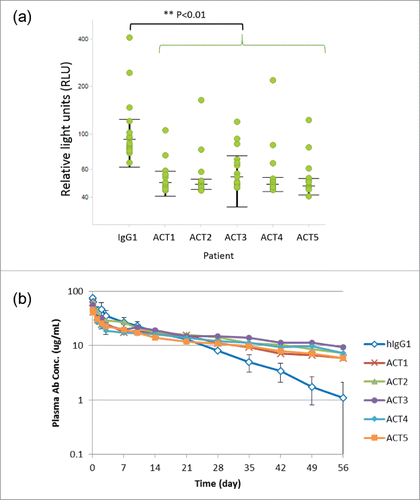
Figure 4. Novel Fc variants ACT1 to ACT5 (the ACT series). (a) Dot plots and statistical significance in RF binding assay for ACT variants. Each individual response of RA patients is shown with median and interquartile range (+/− IQR). Every ACT variant showed significant lower RF binding than intact IgG1 (p<0.01). (b) Time course of plasma antibody concentration in a cynomolgus monkey pharmacokinetic study. Test antibodies have an Fc region of intact human IgG1 (hIgG1) or novel variants (ACT series). The data of several monkeys who showed rapid clearance of a test antibody and were suspected to develop ADA are excluded from the result.