Figures & data
Figure 1. Schematic representation of the PBPK model. The whole body representation shows the included tissues. After injection, antibodies are distributed to the tissues by the blood flows (black arrows). Extravasation into the interstitial space occurs mainly by convection. Alternatively, the antibody can be taken up by the endosome following passive uptake. From the interstitial space, the antibody is then returned to the systemic circulation by the lymphatic system (dashed arrows). In the endosome, the antibody can bind to the FcRn receptor and be recycled to the plasma or, if it does not bind, it might undergo degradation in the lysosome. Upon degradation, the radioactive label might leave the cell fairly quickly in case of a non-residualizing (I-125 / yellow star) label or be trapped in the cell for a certain time in case of a residualizing label (e.g., In-111 / red star). This difference is a good indicator of the extent of degradation.
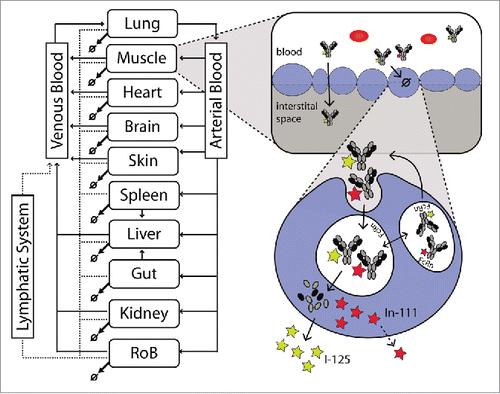
Table 1. Estimated parameters.
Figure 2. Individual plasma PK data of FcRn WT (circles) and FcRn− (triangles) anti-gD antibody are overlaid with the model predicted plasma PK.
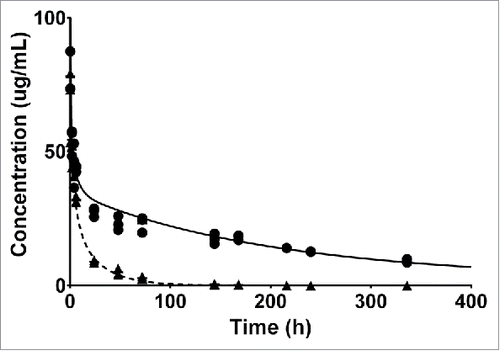
Figure 3. Represents the PBPK fit for all tissue PK data for the FcRn binding antibody. Each tissue is depicted in a subplot, including the measured data and model simulation for I-125 (white circles/gray) and In-111 (black circles/line). The gray shaded area indicates the difference between the measured PK of the I-125 and In-111 labeled antibody and is an indication of the degradation, and subsequent In-111 accumulation in the respective tissue.
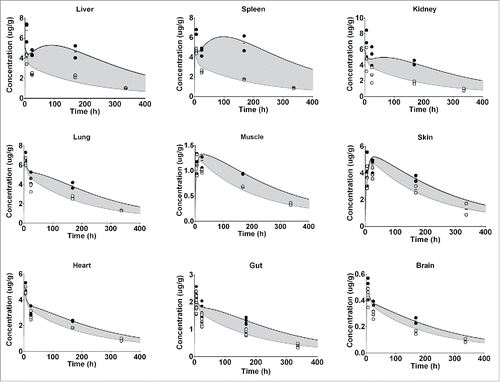
Figure 4. Represents the PBPK fit for all tissue PK data for the FcRn− antibody. Each tissue is depicted in a subplot, including the measured data and model simulation for I-125 (white circles/gray line) and In-111 (black circles/line). The gray shaded area indicates the difference between the measured PK of the I-125 and In-111 labeled antibody and is an indication of the degradation, and subsequent In-111 accumulation in the respective tissue.
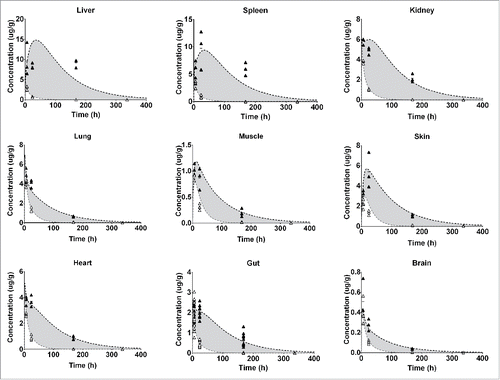
Figure 5. A PBPK-based breakup of total tissue concentration into the contributions of the different sub-compartments. Red shows the contribution of residual plasma, blue of the interstitial space and green of the In-111 label retention. We show 4 representative examples for the different tissues: (a) Gut, which has a quite even contribution of the 3, (b) Liver which is highly degrading and therefore has a high contribution of the retained In-111 label and a quite high residual blood contribution, (c) Lung has high residual plasma, similar to liver, and fairly low interstitial contribution but less label retention and (d) Skin, where the total tissue concentration is mostly based on the interstitial contribution with only very little residual plasma and some label retained after degradation.
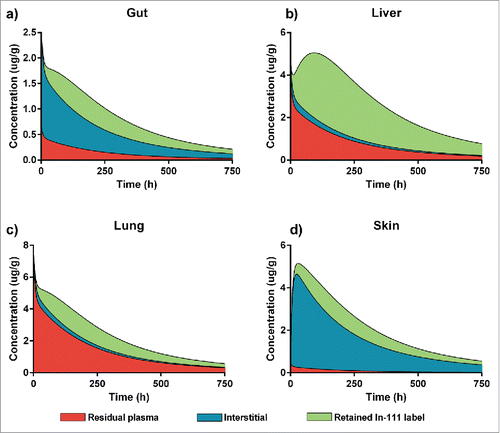
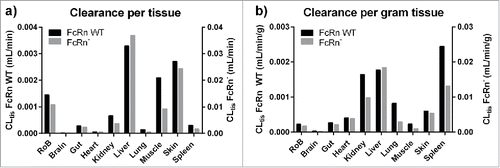
Table 2. Tissue clearances.