Figures & data
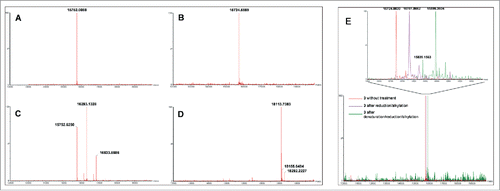
Figure 1. MS analyses of compounds involved in random (A, C) and site-specific approaches (B, D). Analyses (deconvoluted spectra) of starting VHHs 1 (expected Mr = 15,752.3949) (A) and 3 (expected Mr = 15,724.2820) (B), and their respective DOTA/Gd conjugates 2a (expected Mr = 16,293.0421 (DOTA/Gd)1, 16,833.6735 (DOTA/Gd)2) (C) and 5 (expected Mr = 18,113.0720) (D) showed the polydisperse mixture obtained with 2a as opposed to the well-defined conjugate 5. MS analyses of R3VQ-SH 3 showing the presence of a single reduced cysteine and of a stable disulfide bond (E). Analyses (deconvoluted spectra) were realized on 3 without treatment (expected Mr = 15,724.2820), after reduction/alkylation (expected Mr = 15,781.3339 with 1 alkylated cysteine), and after denaturation/reduction/alkylation experiments (expected Mr = 15,895.4378 with 3 alkylated cysteines). The magnified overlay (top) showed the shifts due to alkylation of the thiol functions depending on conditions.
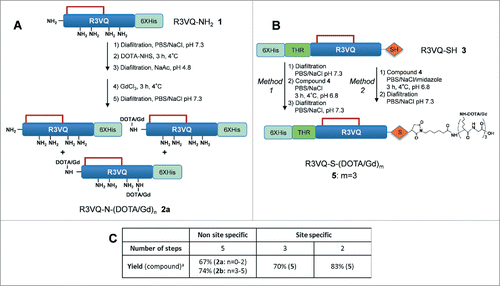
Scheme 1. Overview of random (A) and site-specific (B) chemical conjugationa. aReagents and conditions: (a) Diafiltration, PBS/NaCl, pH 7.3; (b) DOTA-NHS, 3 h, 4 °C; (c). Diafiltration, NaAc, pH 5; (d) GdCl3, 3 h, 4 °C; (e) Compound 4, PBS/NaCl, 3 h, 4 °C, pH 6.8. The site-specific conjugation (B) was performed with (method 1) or without (method 2) initial buffer exchange. Synthesis of compound 4 is described in . Table C summarizes the number of steps and the overall yield of each approach (include all the synthetic process from the starting protein in the affinity column elution buffer). n = average amount of DOTA/Gd per VHH (randomly distributed on different sites). m = exact amount of DOTA/Gd per VHH (located on a single site).
Scheme 2. Synthesis of maleimide-(DOTA/Gd)3 4a. aReagents and conditions: (a) piperidine/DMF 20%; (b) Fmoc-Lys(DOTA(OtBu)3)-OH, HATU, DIEA, DMF or Fmoc-Gly-OH, DIC, DMF; (c) 6-Maleimidohexanoic acid, HATU, DIEA, DMF; (d) TFA/H2O/TIS, 95/2.5/2.5, 4 h; (e) Gd(OAc)3, pH 5, 25 min, 95 °C.
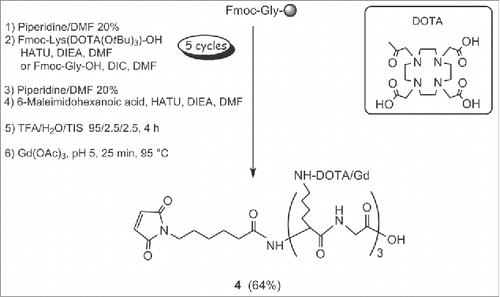
Figure 2. Immunostaining of amyloid deposits by R3VQ-SH 3 (left) and R3VQ-S-(DOTA/Gd)3 5 (right) on brain tissue from mouse model of amyloidosis, showing the preserved properties of the site-specific conjugate (see Figure S7 for IHC controls).
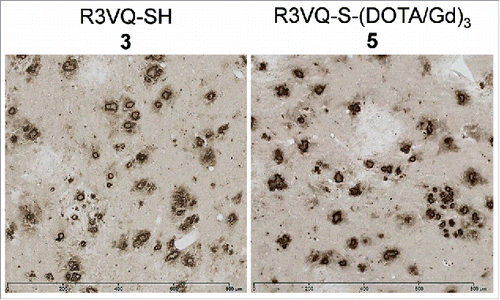
Table 1. ELISA evaluation of antigen binding properties against Aß40 for R3VQ-NH2 1, R3VQ-SH 3 and their respective conjugates 2a, 2b, and 5, highlighting the intact antigen recognition for the site-specific conjugate.
Figure 3. Immunostaining of amyloid plaques after intravenous injection of R3VQ-S-(DOTA/Gd)3 5, highlighting its ability to cross the BBB in vivo. PS2APP mice (15 month-old) were injected in the tail vein with compound 5 at 50 mg/kg (left panel, n = 3) or with PBS (right panel, n = 2), and sacrificed after 4 hours. IHC were realized with an anti-His-tag antibody. Whereas only unspecific background was observed in mice brains injected with PBS, a specific labeling of amyloid deposits was detected in those injected with 5 thus confirming the ability of the conjugate to cross the BBB after in vivo injection.
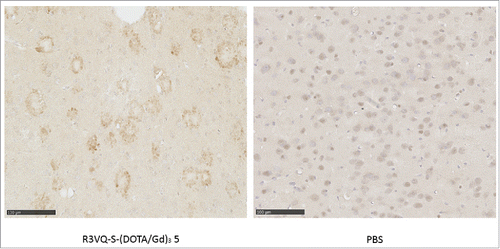
Figure 4. MR relaxometric parameters at three different magnetic fields. Measures of r1 and r2 (normalized per mM of Gd) showed the high relaxivities of the contrast agent R3VQ-S-(DOTA/Gd)3 5 that reach values until 10 times higher than those of the reference contrast agent DOTAREM. Triplicate measures of r1 and r2 (in mM−1.s−1) are expressed as mean +/− SEM (detailed values in Fig. S9).
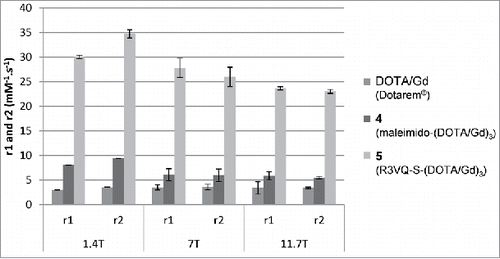
Figure 5. In vitro MRI revelation of amyloid deposits by the contrast agent R3VQ-S-(DOTA/Gd)3 5. (A) PS2APP or wild-type (amyloid free) brains were incubated with PBS or 5 before MRI acquisitions (left frames). IHC were realized with 4G8 antibody as a reference anti-beta amyloid antibody (middle frames), or an anti-His-tag antibody to reveal the amyloid deposits labeled by the contrast agent 5 (right frames). MRI acquisitions were performed with a 25 µm isotropic resolution (n = 2/group). Red squares show the magnified areas used for the registration. Scale bar = 500 µm. See for hypointense spots quantification. (B) Registration was done between MRI, 4G8 and anti-His-tag IHC on PS2APP tissues incubated with 5. Hypointense spots on MR images correspond to amyloid deposits labeled by 5 on the anti-His-tag IHC and by 4G8 (red arrows). White dotted lines represent landmarks that delimited the corpus callosum and the hippocampus. Scale bar = 250 µm.
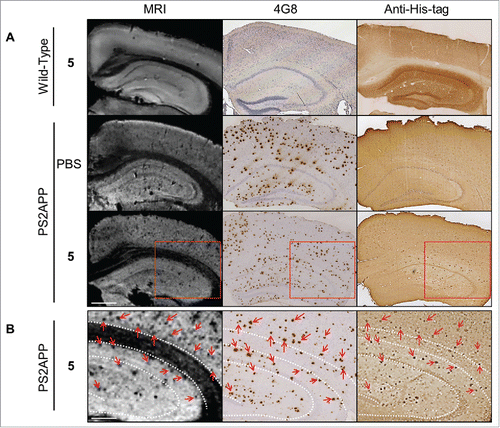
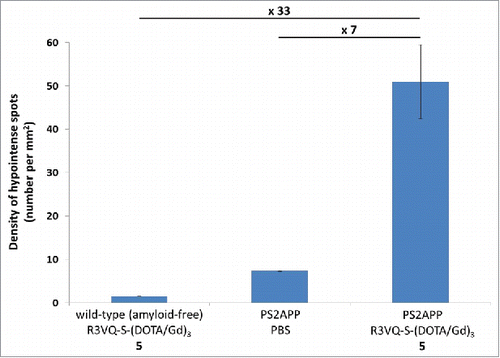
Figure 6. Quantification of hypointense spots detected on MR images. Measures from MR images obtained on post mortem brain tissues () confirmed the major increase in amyloid plaques detection in PS2APP brains incubated with the R3VQ-S-(DOTA/Gd)3 5 compared to the controls, i.e., PS2APP brains incubated with PBS or wild-type amyloid-free brains incubated with R3VQ-S-(DOTA/Gd)3 5.