Figures & data
Table 1. Major revisions to ETN DP manufacturing process.
Table 2. Main Enbrel Quality Attributes and Characterization Tests.
Figure 1. Purity levels by HIC HPLC (Peak 3: levels of aggregated and misfolded species) (a); impurity levels as HCP by ELISA (b); binding activity to TNF by ELISA (c); and potency by inhibition of TNF-induced apoptosis in a cell-based bioassay (d), of ETN DP batches (1998–2015) across 3 manufacturing sites according to manufacturing process (A–D)* DP, drug product; DS, drug substance; ELISA, enzyme-linked immunosorbent assay; ETN, etanercept; HIC, hydrophobic interaction chromatography; HCP, host cell proteins; HPLC, high-performance liquid chromatography; TNF, tumor necrosis factor.
Minor variations in the number of batches included per process across each test method reflect minor differences in investigative or repeat testing. *Data (horizontal lines) are median values (95% confidence interval) and interquartile range; mean values are indicated by the square symbol. The dotted lines indicate the specification limits for ETN DS.
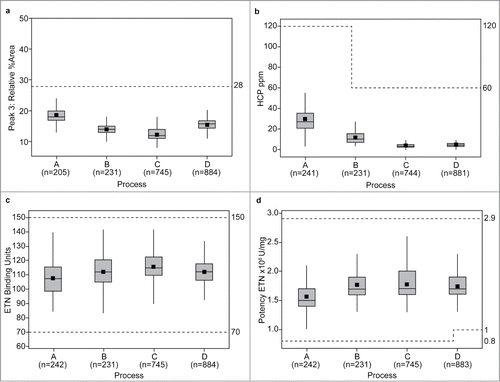
Figure 2. Relative abundance of neutral species (Peaks 1, 2/3, 4, and 5) (a); sialylated species (Peaks 6–9) (b); and core fucosylated (Peaks 1, 2/3, 5, 7, and 9) N-linked oligosaccharides (c), across process variations (A–D). Specifications for individual peaks were introduced following implementation of process D. Data available to create statistical representations therefore differ compared with those in . *Data (horizontal lines) are median values and interquartile range. The whiskers indicate the highest and lowest data values within the upper and lower limits, respectively. Mean values are indicated by the square symbol.
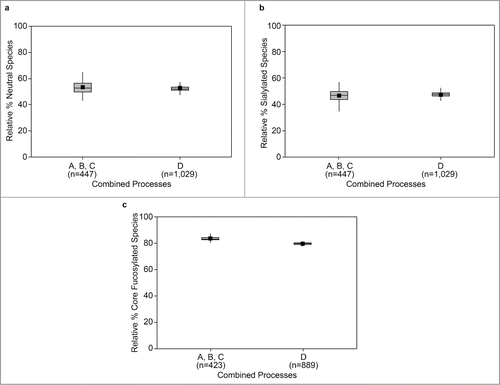
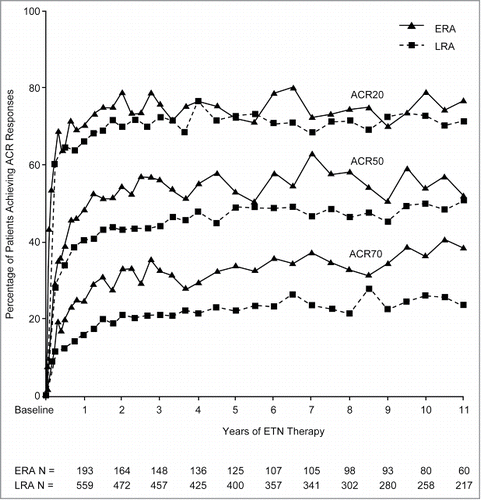
Figure 3. Sustained improvement in American College of Rheumatology (ACR) response (Reprinted with permission from Weinblatt et al., “Safety and Efficacy of Etanercept Beyond 10 Years of Therapy in North American Patients With Early and Longstanding Rheumatoid Arthritis” Arthritis Care & Research Vol. 63(3), 373–382, Wiley. © 2011, American College of RheumatologyCitation17)*, *Percentages of early rheumatoid arthritis (ERA) patients and longstanding rheumatoid arthritis (LRA) patients who achieved ACR criteria for 20%/50%/70% improvement in disease activity (ACR20/50/70) responses from baseline throughout year 11 are shown. ACR data are reported using C-reactive protein (CRP) values based on the enzyme immunoassay (EIA) of CRP (ERA: months 1–78, LRA: months 1–60), the average of the EIA and the new high-sensitivity method (ERA: months 84–90, LRA: months 66–90), and the high-sensitivity method (ERA and LRA: months ≥96). Numbers of ERA and LRA patients evaluated during each year are indicated. Values were not available for all patients at all time points.