Figures & data
Table 1. Asthma biomarkers used in clinical trials to predict the response to biologics directed at mediators of type 2 asthma.
Table 2. Characteristics of type 2 and non-type 2 airway inflammations in asthma.
Figure 1. Simplified schematic representation of four different types of airway inflammation in asthmatic patients. (A) Type 2 consists of allergic and nonallergic eosinophilic asthma. (a) In allergic eosinophilic asthma, T-helper type 2 (TH2) cell lymphocytes and mast cells drive eosinophilic airway inflammation in an allergen-specific, immunoglobulin E (IgE)-dependent manner. (b) In nonallergic eosinophilic asthma, innate lymphocytes such as natural killer T cells (NKT cells) and innate lymphoid cells type 2 (ILC2) cells might contribute to airway eosinophilia via the production of interleukin (IL)-5, in response to pollutants or infectious agents. (B) Non-type 2 consists of neutrophilic and paucigranulocytic asthma. (c) The mechanisms underlying neutrophilic asthma need to be elucidated, but the IL-17 pathway and CXCL8 have been associated with the airway neutrophilia. More precisely, IL-17A and IL-17F play important roles in host responses to extracellular pathogens via the upregulation of antimicrobial proteins and induction of cytokines and chemokines involved in neutrophil expansion (e.g., GM-CSF) and recruitment (e.g., CXCR ligands). (d) Paucigranulocytic asthma has been poorly studied. It is thought to be not inflammatory and is characterized by the absence of increased numbers of inflammatory cells, suggesting the involvement of non-inflammatory mechanisms mediated by airway remodeling responses that lead to extensive airway narrowing. The biologics being evaluated in clinical trials or already approved as add-on treatment, on top of high-doses inhaled corticosteroid (ICS) and a short- or long-acting β2-adrenergic agonist (LABA), for (C) allergic and (D) nonallergic eosinophilic asthma are depicted in light grey. CRTH2: prostaglandin D2 receptor 2; CXCL8: C-X-C motif chemokine ligand 8; CXCR: C-X-C chemokine receptor; EGF: epidermal growth factor; EGFR: epidermal growth factor receptor; FcϵRI: Fc epsilon receptor I; GM-CSF: granulocyte-macrophage colony-stimulating factor; ICS: inhaled corticosteroid; IgE: immunoglobulin epsilon; ILC2: innate lymphoid cell type 2; IL: interleukin; IL-4Rα: interleukin-4 receptor alpha; IL-5Rα: interleukin-5 receptor alpha; IL-9R: interleukin-9 receptor; IL-25R: interleukin-25 receptor; IL-33R: interleukin-33 receptor; LABA: long-acting β2-adrenergic; MHC: major histocompatibility complex; NKT: natural killer T; PGD2: prostaglandin D2; TCR: T-cell receptor; TH2: T-helper type 2 cell; TH17: T-helper type 17 cell; TSLP: thymic stromal lymphoietin; TSLPR: thymic stromal lymphoietin receptor.
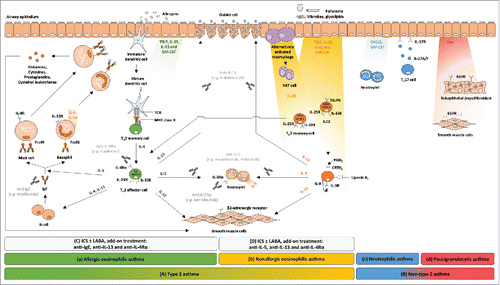
Table 3. Biologics evaluated for the treatment of moderate-to-severe type 2 asthma.
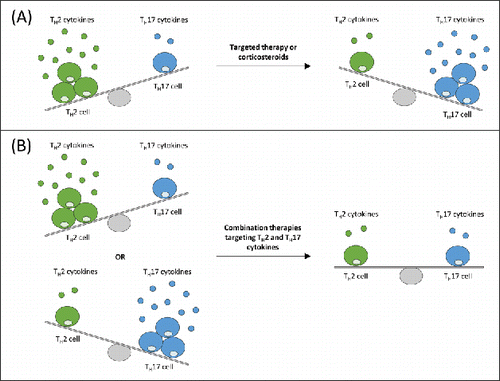
Figure 2. Simplified schematic representation of targeted TH2 and TH17 cytokines therapies that can lead to amplification of activity of the opposing pathway in a murine house dust mite model of asthma.Citation73 (A) With suppression of TH2 activity by targeted therapy or corticosteroids, a TH17-permissive environment exists. A direct relationship between TH17 and TH2 disease exists, whereby, through mutual cross regulation, TH17 asthma may represent a transition or switch away from TH2-mediated disease. Thus, by treating TH2 patients with corticosteroids, TH17 asthma may have been promoted. (B) Combination therapy targeting TH2 cytokine, such as interleukin-13, and TH17 cytokine, such as interleukin-17, in patients expressing either a TH2 or TH17 signature may provide additional efficacy over single TH2 or TH17 inhibition. TH2: T-helper type 2 cell; TH17: T-helper type 17 cell.