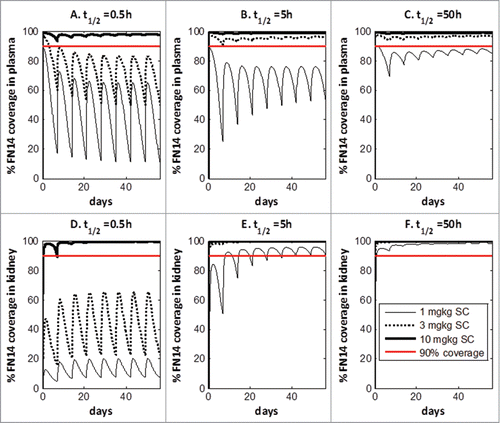
Figures & data
Figure 1. The mechanistic SoA model developed for predicting target coverage on membrane Fn14 in kidney in the presence of circulating soluble Fn14.
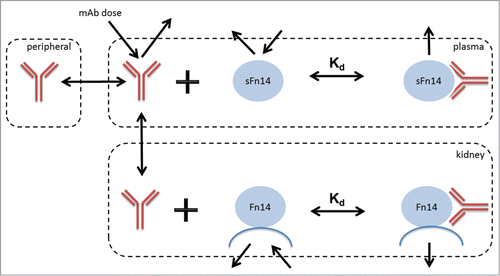
Figure 2. Experimental measures for mFn14 and sFn14 levels in human. Left bar: concentration of mFn14 in human kidney tissue from diabetic nephropathy patients. Right bar: concentration of sFn14 in human serum from healthy subjects. The bar height represents the mean value and error bar represents the standard deviation. The y-axis is in log scale.
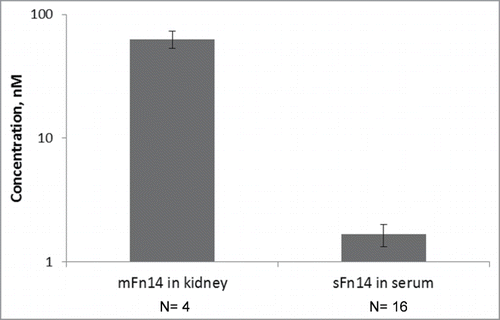
Figure 3. Pulse-chase experiment for determining serum turnover rate of sFn14. a: Chromatograms at selected time points showing the growth and decay of the newly synthesized peptide (blue) ratio to light peptide (red). b: The enrichment of heavy leucine (▪) and heavy sFn14 peptide (▴) in human serum and the corresponding fitted curves. The graph is from one representative subject. Accordingly, the calculated average half-life of sFn14 was 5 ± 0.5 hours in three human subjects.
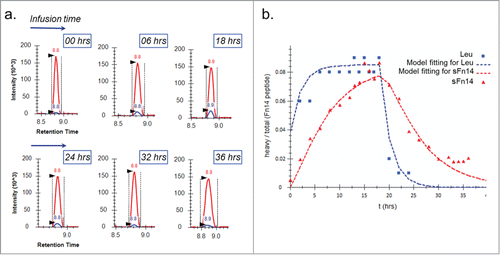
Table 1. Model parameters for mechanistic SoA model.
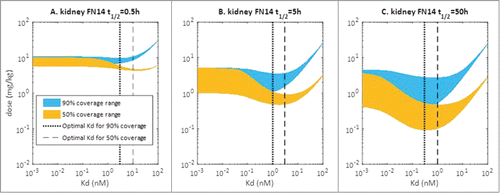
Figure 4. Simulated relationship between required dose and Kd of anti-Fn14 mAb as a function of 1) mFn14 half-life in kidney (0.5, 5, or 50 hours as illustrated in panels), 2) pre-specified target coverage (90% or 50% as illustrated as blue or yellow curve), and 3) sFn14 concentrations (baseline level in healthy subjects to 10 times baseline, as expressed in width of bands). Optimal Kd of anti-Fn14 mAb is the Kd at which the required dose is the lowest (illustrated as vertical lines).