Figures & data
Figure 1. Treatment schematic, pain assessment and intravenous morphine usage during LTI of ch14.18/CHO. A) Ch14.18/CHO was administered by LTI of 100 mg/m2 (d8–17) (horizontal bar) with 6 × 106 IU/m2 s.c. IL-2 (d1–5; 8–12) (black arrows) and p.o. isotretinoin (160 mg/m2/day d22–35). Pain toxicity of anti-GD2 antibody ch14.18/CHO was evaluated by systematic assessments of pain scores and intravenous morphine usage of 49/53 evaluable patients as described in the “Patients and Methods” section. B) Pain assessment scores were determined three times daily per patient and cycle. Data represent mean ± SEM. C) Usage of i.v. morphine in µg/kg/h was determined daily per patient and cycle and presented as mean ± SEM. When error bars are not visible, they are covered by the symbol. Total morphine usage per cycle ± SEM is indicated in mg/kg/cycle.
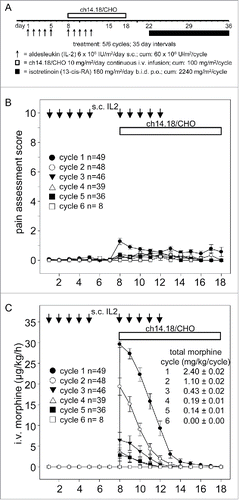
Figure 2. Analysis of survival and time to progression following LTI of ch14.18/CHO. Patients treated by LTI of 100 mg/m2 ch14.18/CHO in combination with 6 × 106 IU/m2 s.c. IL-2 (d1–5; 8–12) and oral 13-cis RA (d19–32) were analyzed for progression-free survival (PFS) (A) and overall survival (OS) (B) using the Kaplan-Meier method. Patients of the entire cohort (n = 53) and patients with relapsed status at base line (n = 29) were analyzed separately. A) PFS curves of the entire LTI cohort (red) and relapsed patients (blue) (top panel). B) OS of the entire LTI cohort (red) and relapsed patients (blue) was compared to relapsed patients of the AIEOP data base not treated with ch14.18/CHO (green).Citation21 The starting point of the AIEOP relapsed patients equals to the date of first relapse plus the median time between relapse and start of ch14.18/CHO therapy for the LTI patients (1 y 7 d). Patients in the AIEOP relapsed group who died before the auxiliary starting point were excluded. The difference between LTI relapsed- and AIEOP relapsed- patients was statistically significant (P = 0.002).
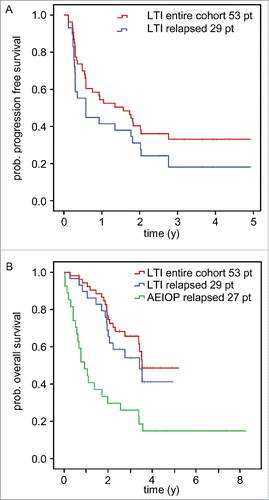
Table 1. Demographics and characteristics of high-risk NB patients treated by long term infusion (LTI) of ch14.18/CHO at initial diagnosis and at the time of LTI treatment start.
Table 2. Treatment Emergent Adverse Events (TEAEs) of Grade ≥3 related to ch14.18/CHO LTI combined with IL-2.
Table 3. Treatment response in 37 evaluable relapsed/refractory patients with measurable disease at baseline.
Table 4. Demographics and baseline characteristics of relapsed NB patients evaluable for historical comparison.
