Figures & data
Figure 1. Off-target binding analysis chip re-array assay.
After performing an array-based binding screen on 4,975 human receptors for SHR-1210-IgG1, confirmatory analyses of binding specificity were performed on chips in which plasmids encoding PD1 and putative SHR-1210 off-target binding proteins were arrayed and used to transfect HEK-293 cells. Effective transfection of all plasmids was confirmed by screening for the co-encoded marker ZS green (A). Separate chips were then probed in duplicate using SHR-1210-IgG1 (B), Isotype IgG1 (C), Rituximab (D), and no primary antibody (E). These analyses confirmed that only SHR-1210-IgG1 exhibited binding to PD1, but also exhibited unexpected off-target binding to VEGFR2, FZD5 and ULBP2 proteins.
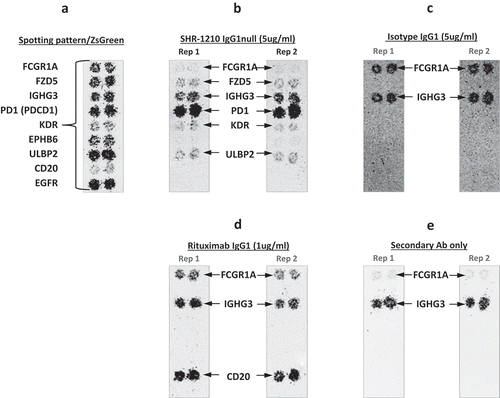
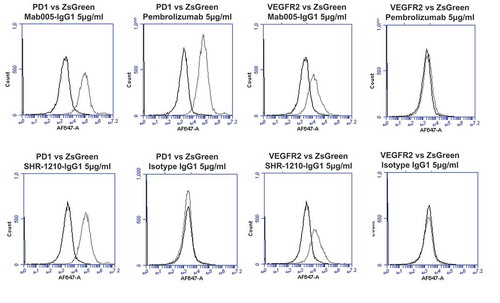
Figure 2. Specificity analyses by flow cytometry using transiently-transfected HEK-293 cells.
Analyses of binding specificity were performed on HEK-293 cells transiently transfected with plasmids encoding either (A) human PD1, human VEGFR2, (B) human FZD5, human ULBP2. All plots show the target of interest transfected (grey line) versus ZS green marker-only transfected cells (black line). Transfected cells were stained using Mab005-IgG1, SHR-1210-IgG1, Pembrolizumab IgG1 null analog, and isotype IgG1. Each antibody was used in repeat staining at 5 μg/ml. These analyses confirmed that all antibodies (other than the isotype control IgG1) exhibited binding to PD1, but no antibody exhibited measurable signal on ZS-green transfected cells. Both Mab005-IgG1 and SHR-1210-IgG1 also exhibited strong binding to all targets, while the isotype IgG1 and Pembrolizumab analog did not.
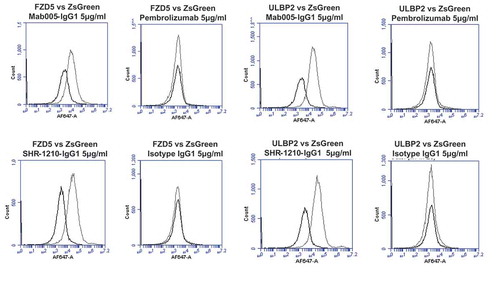
Table 1. Amino acid sequence of MAB005 murine anti-PD1 v-domains (mVH/mVL) and SHR-1210 CDR grafts.
Figure 3. Analysis of CDR residue tolerance for mutation to germline.
A plot of murine amino acid retention frequencies in the CDRs of the ELISA-positive population of 64 unique Fab fragment clones is shown for VL (A) and VH (B) domains, respectively. Only those residues targeted for human/murine residue mutagenesis are plotted, other than in the HCDR3. CDR residues noted in parentheses on the X-axes were identical to those found in the human germlines used for grafting (IGKV1-39 and IGHV3-7). In both plots the dashed line in grey at 75% represents the cut-off for tolerance of murine residue replacement by human germline.
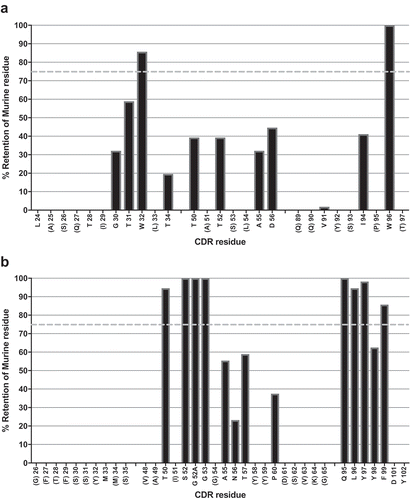
Table 2. Biacore affinity values for IgG1null binding to human and cyno monomeric PD1.
Figure 4. Direct titration ELISA for library-derived IgG1null clones binding to human and cyno PD1-Fc proteins.
SHR-1210-IgG1, library-derived clones (A, B) and designer clones (C-F) in human IgG1null format were titrated (in nM) in a direct binding ELISA against human and cyno PD1-Fc proteins.
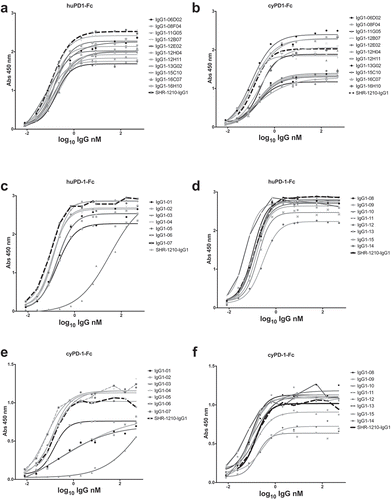
Figure 5. SHR-1210 epitope competition analysis of IgG1null proteins in Alphascreen.
Anti-PD1 IgG1null clones were applied in an epitope competition assay using Alphascreen technology. In this assay, library-derived (A) and designer (B) IgGs were analysed for their relative affinities and retention of the parental SHR-1210 epitope by competing for SHR-1210-IgG1 binding to human PD1 protein, in solution. All clones analysed showed strong, concentration-dependent neutralization of SHR-1210-IgG1 binding to PD1, indicating maintenance of the same binding epitope.
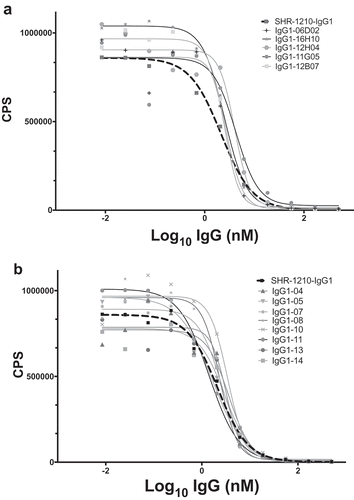
Table 3. EC50 values for IgG1null binding to human and cyno PD1-CHO cells.
Figure 6. Flow cytometric binding to human and cyno PD1+ CHO cells.
SHR-1210-IgG1, lead library-derived (A) and designer (B) IgGs were examined for specific binding on CHO-K1 cells expressing human PD1. SHR-1210-IgG1, lead library-derived (C) and designer (D) IgGs were also examined for specific binding on CHO-K1 cells expressing cyno PD1. Concentration-dependent binding was observed against human and cyno PD1 for all clones, with weaker binding being observed for SHR-1210-IgG1 in each experiment.
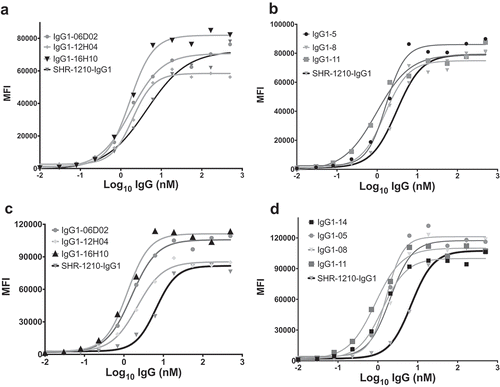
Table 4. EC50 values for IgG1null blockade of human PD1/PD-L1.
Figure 7. Cell-based PD1/PD-L1 antagonism assay.
Analyses of antagonism of human PD1 function at the cell surface, for example lead clones IgG1-11, IgG1-14, IgG1-06D02 and IgG1-16H10 in human IgG1null format, showed that all novel clones exhibited concentration-dependent antagonistic activity, with higher relative potency in comparison to both SHR-1210-IgG1 and IgG4 nivolumab analog.
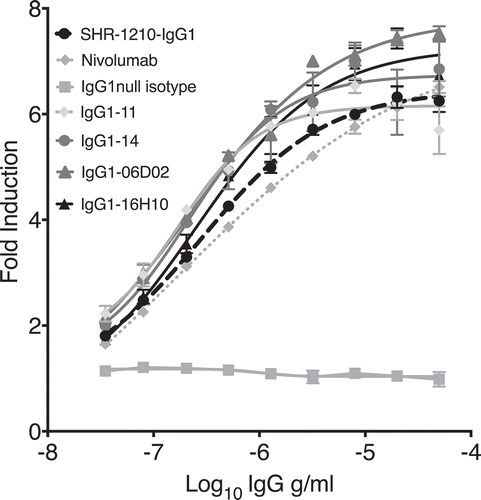
Table 5. Human T cell epitope content in v-domains predicted by iTOPETM and TCEDTM.
Figure 8. Off-target binding analysis re-array assay.
Analyses of binding specificity were performed on chips in which plasmids encoding relevant targets were arrayed and used to transfect HEK-293 cells. Transfection of all plasmids was confirmed by screening for the co-encoded marker ZS green (A). Separate chips were then probed in duplicate using pembrolizumab analog (B), rituximab (C), Isotype IgG1 (D), SHR-1210-IgG1 (E), and example library-derived and designer lead IgGs (F-L). These analyses confirmed that all anti-PD1 antibodies exhibited binding to PD1, but only SHR-1210-IgG1 also exhibited unexpected off-target binding to VEGFR2, FZD5 and ULBP2 proteins.
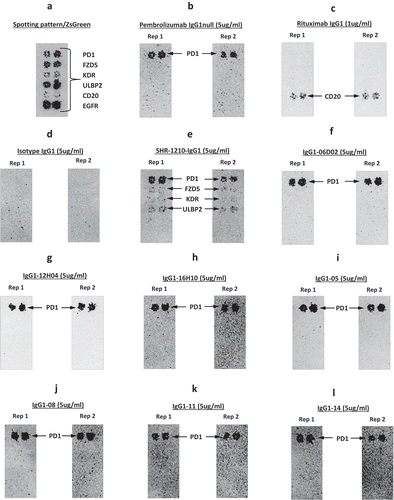
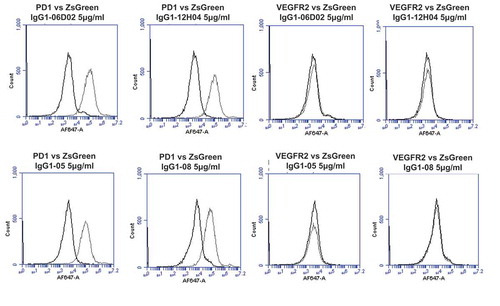
Figure 9. Specificity analyses for library-derived and designer lead IgGs by flow cytometry using transiently-transfected HEK-293 cells.
Analyses of binding specificity were performed on HEK-293 cells transiently transfected with plasmids encoding either (A) human PD1 and human VEGFR2, (B) human FZD5 and human ULBP2. All plots show the target of interest transfected cells (grey line) versus ZS green marker-only transfected cells (black line). Each antibody was used in repeat staining at 5 μg/ml. These analyses confirmed that all antibodies (other than the isotype control IgG1) exhibited binding to PD1, but no antibody exhibited measurable signal on ZS-green, VEGFR2, FZD5 or ULBP2 transfected cells.
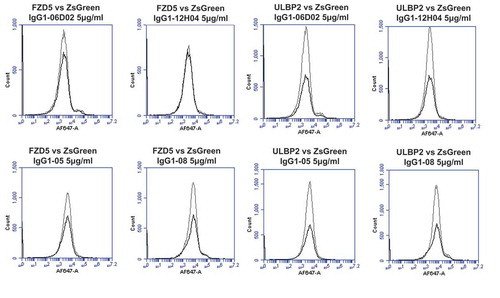
Figure 10. Off-target binding analysis ELISA assay.
SHR-1210-IgG1, Mab005-IgG1, library-derived clones and designer clones in human IgG1null format were titrated (in μg/ml) in a direct binding ELISA against human PD1 (A) and cyno PD1 (B), human VEGFR2 (C) and rhesus VEGFR2 (D), human FZD5 (E) and BSA (F) proteins. These analyses confirmed that all anti-PD1 antibodies exhibited binding to PD1, but only Mab005-IgG1 and SHR-1210-IgG1 exhibited measurable off-target binding to VEGFR2 and FZD5 proteins.
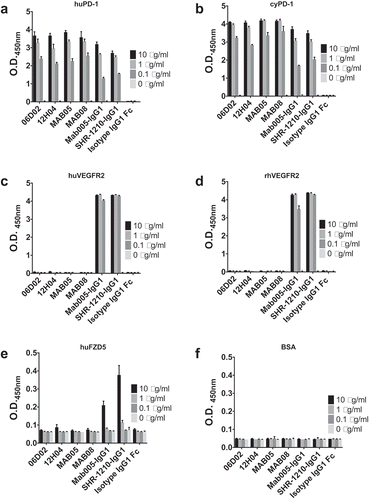
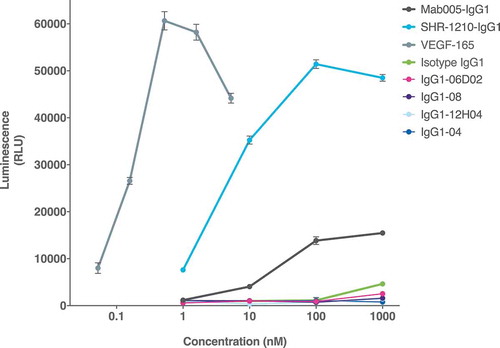
Figure 11. Cell-based VEGFR2 agonism assay.
SHR-1210-IgG1, Mab005-IgG1, library-derived clones and designer clones in human IgG1null format were titrated (in nM) in a human VEGFR2 signalling assay. SHR-1210-IgG1, Mab005-IgG1 and the positive control protein (human VEGF-165) all induced strong, concentration-dependent VEGFR2 agonism. Lead clones IgG1-04, IgG1-08, IgG1-06D02, and IgG1-12H04 showed no measurable agonism, even at concentrations as high as 1 μM.