Figures & data
Figure 1. Tetravalent Db-Fc fusion proteins. (a) the schematic construction of a heavy and light chain of Db-Ig molecules. Variable domains are fused via G4S linker to build a diabody as binding region. The dimerization domain (DD1 and DD2) originate from either a heterodimer (CH1/CL or hetEHD2) or a homodimer (EHD2 or MHD2). The Fc part is formed by the hinge region and CH2/CH3 of an IgG. (b) schematic illustration of a tetravalent Db-Ig molecule with symmetric architecture. In dependence of the variable domains, this molecule can be either mono- (FvA = FvB) or bispecific (FvA≠FvB). N-glycans are shown as black pentagons. (c) Schematic illustration of tetravalent Db-Ig molecules using either homodimerization domains (EHD2 or MHD2) or heterodimerization domains (CH1/CL or hetEHD2). N-glycans are shown as black pentagons.
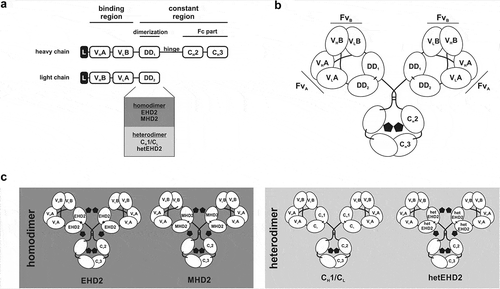
Figure 2. Biochemical characterization of Db3-43xhu225-Ig molecules. (a) Schematic illustration of Db3-43xhu225-Ig molecules using CH1/CL, EHD2, MHD2, or hetEHD2 as dimerization module. (b) SDS-PAGE analysis of Db3-43xhu225-Ig molecules under reducing (1) or non-reducing (2) conditions (4–12% PAA gradient). Proteins were stained with Coomassie blue. (c) Size-exclusion chromatography of Db3-43xhu225-Ig molecules by HPLC using a Tosoh TSKgelSuperSW column.
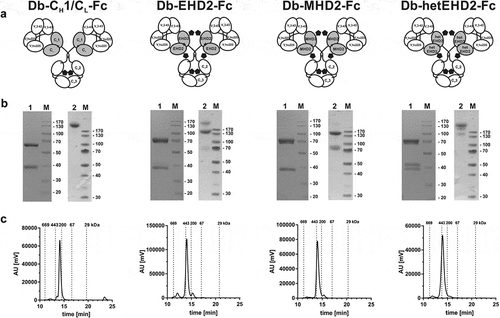
Table 1. EC50 values [pM] of bispecific Db3–43xhu225-Ig molecules determined by ELISA binding experiments or flow cytometry analysis. Mean ± SD, n = 3.
Figure 3. Binding of different Db3-43xhu225-Ig molecules to EGFR and HER3 by ELISA. A + B Binding of Db3-43xhu225-Ig molecules to immobilized EGFR-His (a) or HER3-His (b). Parental antibodies IgG hu225 and IgG 3-43 were included as a control. Bound antibodies were detected with an HRP-conjugated anti-human Fc secondary antibody. Mean ± SD, n = 3. (c) Simultaneous binding of both antigens was analyzed using EGFR-Fc as immobilized antigen. Serial dilution of different antibodies was added to the wells. Finally, the second antigen, HER3-His, was added to the wells and bound HER3-His was detected using an HRP-conjugated anti-His secondary antibody. Mean ± SD, n = 3.
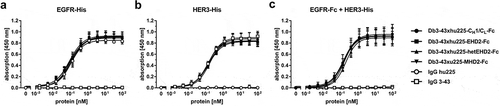
Figure 4. Binding and bioactivity of bispecific Db3-43xhu225-Ig molecules on FaDu cells. (a) Binding of different antibodies to FaDu cells was analyzed by flow cytometry. Bound antibodies were detected using a PE-labeled anti-human Fc secondary antibody. Mean ± SD, n = 3. (b) Proliferation assay of different bispecific Db3-43xhu225-Ig molecules (50 nM) using FaDu cells in starvation medium after incubation of 7 days. Cells were kept either unstimulated (w/o ligand) or stimulated with heregulin (HRG). Parental antibodies were included as control (single treatment: 50 nM; combination: 50 nM each). Cell viability was measured using CellTiter-Glo 2.0. Mean ± SD, n = 3. (c) Flow cytometry analysis of annexinV/PI staining using FaDu cells. Bispecific Db3-43xhu225-CH1/CL-Fc molecule (50 nM), or parental antibodies (single treatment: 50 nM; combination: 50 nM each) were incubated with cells in complete medium for 24 h. Mean ± SD, n = 3. *p < .05; **p < .01; ***p < .001; ****p < .0001.
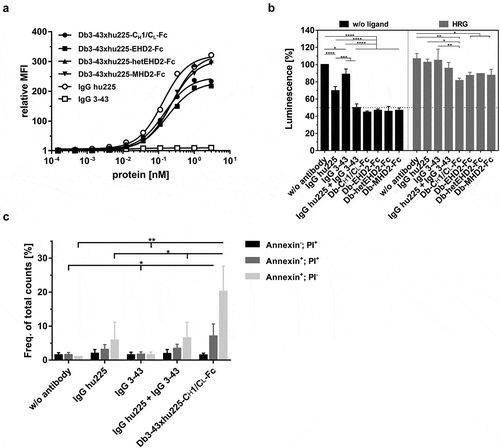
Table 2. Determination of initial (t½α) and terminal (t½β) half-lives as well as of drug exposure (area under the curve; AUC) of bispecific Db3-43xhu225-Ig molecules. Mean ± SD, n = 3.
Figure 5. Plasma stability and PK analysis of bispecific Db3-43xhu225-Ig molecules. (a) The Db-Ig molecules were diluted in 50% human plasma and incubated at 37°C for, 1, 3, or 7 days. Protein content was determined by ELISA using both antigens, EGFR-His or HER3-His. (b) Pharmacokinetic profile of Db-Ig molecules were analyzed in female CD-1 mice (n = 3). Mice received a single i.v. injection of 25 µg of the protein. Serum protein concentrations were determined by ELISA using both antigens, EGFR-His or HER3-His.
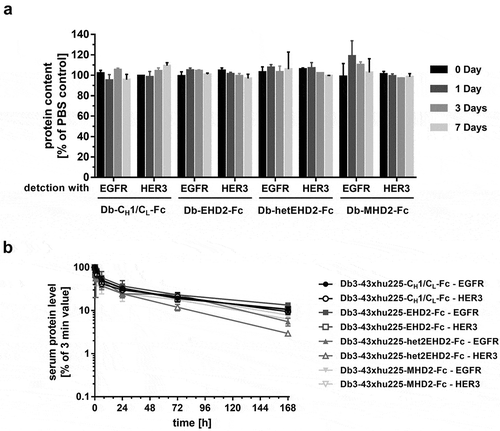
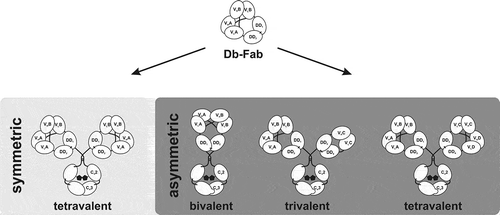
Figure 6. Schematic overview of symmetric and asymmetric Db-Ig molecules. Symmetric tetravalent molecules are designed by fusing a wild-type Fc part to one chain of the Db-Fab arm resulting in mono- (VA = VB) or bispecific (VA≠VB) antibodies. By introducing a heterodimeric assembled Fc part (e.g., knob-into-hole), asymmetric molecules can be engineered, which can be bivalent (1 + 1), trivalent (3 + 0; 2 + 1; 1 + 1 + 1), or tetravalent (2 + 2; 2 + 1 + 1; 1 + 1 + 1 + 1).