Figures & data
Figure 1. Generation of CHO-GS−/- cells using TALENs DNA-editing technology. (a) Generation and characterization of TALENs targeting CHO GS. A suitable TALEN target site was found in exon 6 of the GS gene with the TALEN binding sites separated by a spacer of 15-bp. Graphic was generated with the aid of CRISPy software.Citation28 TALENs each with 18.5 RVDs and 20-bp specificity were assembled by Golden Gate Cloning. The first 5ʹ T of each binding site is recognized by the N-terminus of TALEN protein. The NH monomer was used to recognize G for increased specificity. (b) T7E1 mismatch assay indicates that the designed GS TALENs has gene modification activity of 25.8%. (c) DNA sequence analysis of the GS gene from CHO WT cells and CHO-GS−/- cells for clones 1, 2 and 3. The TALEN binding sites are in red, the 15-bp spacer is in black and underlined. The resulting deletions identified in different CHO mutants are shown on the right. Clone 3 showed two different deletions in the two alleles. (d) Western blot analysis confirms the successful knockout of GS in the CHO cells. Endogenous GS was detected in cell lysates. Whole cell proteins were resolved through SDS-PAGE under reducing conditions, and, after transfer onto PVDF membrane, detected with mouse monoclonal anti-GS antibody and probed with secondary HRP-conjugated goat anti-mouse IgG + IgM (H + L) antibody. Actin was used as a loading control. (e) TALENs-inactivated GS mutants show dependency for glutamine. Cells were seeded overnight in six-well plates at 200 000 cells per well in normal media and replaced with the respective media as indicated. Five days later, cell growth and morphology were observed. Both parental CHO-K1 and GS knockout cells showed normal growth and morphology in normal media. However, in glutamine-deprived media, GS-mutant demonstrated growth-arrest characteristics with rounded and shrunk morphology. On the other hand, CHO-K1 WT cells showed slower growth in glutamine-deprived media, but maintained its characteristic fibroblastic morphology.
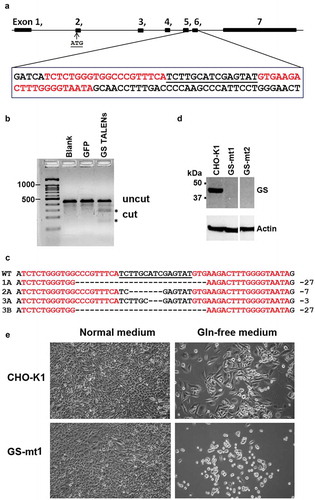
Figure 2. Evaluation of GS R324C as a selection marker for the generation of GFP-expressing stable cells. (a) Comparison of GSwt with congenital mutant R324C on the generation of stable GFP expressing CHO cells. GFP-IRES-GS and GFP-IRES-GS R324C constructs were transfected into GS−/- CHO cells – clones #8, #9, #11, and #13. The GS gene in these clones was previously knocked out with targeted zinc-finger nucleases (data not shown). The resulting stable pools were analyzed for GFP expression using FACSAriaIII. Representative area dot blots for GFP fluorescence vs FSC are shown. (b) GFP mean fluorescence intensity (MFI) is expressed as a mean of biological replicates (n = 2–3 + SD).
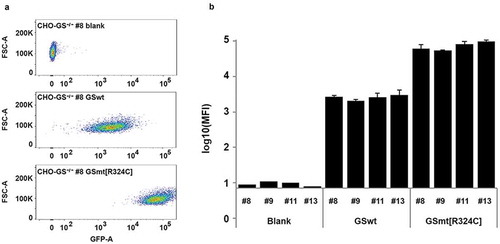
Figure 3. Evaluation of GS R324C as a selection marker for the generation of anti-CD20 antibody GA101 expressing stable cells. (a) Antibody GA101 titers of CHO-GS−/- stable pools have been generated using GSwt or R324C as the selection marker in a bicistronic vector. At least three independent stable pools were generated under each condition in CHO-GS−/- clone 1. The GSwt pools that survived the L-glutamine deprivation selection were further treated with 25 μM MSX (GSwt + MSX) to improve the antibody production. Batch culture titers were analyzed using nephelometer IgG reagent measurement. Stability assessment of single clones isolated from one of the bicistronic GSwt + MSX (b) or R324C (c) stable pools over at least 60 generations for antibody GA101 production. IgG titers were normalized to week 0 (MSX removal) for each clone in glutamine-free media.
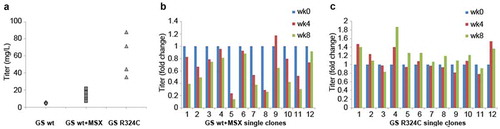
Figure 4. Identification of novel GS residues critical to GS activity. (a) Alignment of human and Chinese hamster GS protein sequences. Conserved sites involved in ammonium, glutamate and ATP binding are highlighted in green, red and blue as depicted together with their residue number.Citation27 The congenital disease mutations R324C and R341C are pointed out with the black arrowheads. R341C residue is not involved in neither the ammonium, glutamate nor ATP binding, and is highlighted in yellow. (b) Comparison of GSwt activity with the various alanine mutants of the conserved residues highlighted in (a). The GS activities were normalized to luciferase activity to minimize expression level variations. Each GS construct was linked to a luciferase gene via IRES in a bicistronic manner. CHO-GS−/- clone 1 cells were transiently transfected with the constructs and total cell lysates were harvested. The GS activities were evaluated using the standard GS activity assay whereby GS–catalyzed formation of γ -glutamylhydroxamate from glutamine and hydroxylamine was measured photometrically at 500 nm. The activities of the mutants were represented as fold-change to GSwt.
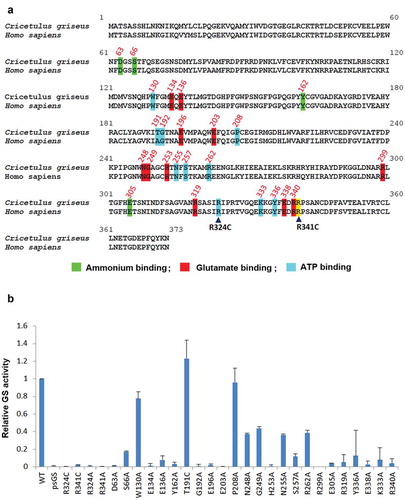
Figure 5. Comparison of the novel GS mutants to GSwt as a selection marker in generating stable antibody-producing cell lines. (a) Comparison of the antibody GA101 titer production in CHO-GS−/- clone 1 stable pools generated using GSwt, GSwt + 25 µM MSX, and the various GS mutants (as labeled) as the selection condition. The antibody GA101 was expressed in a tricistronic vector configuration. (b) Stability assessment of antibody titer from single clones isolated from one of the GSwt + MSX stable pools over at least 60 generations. Titers were normalized to week 0 (MSX removal) for each clone in L-glutamine-free media. (c) Stability assessment of antibody titer from single clones isolated from one of the GS (D63A) stable pools over at least 60 generations. Titers were normalized to week 0 for each clone in L-glutamine-free media. (d) Stability assessment of antibody titer from single clones isolated from one of the GS (S257A) stable pools over at least 60 generations. Titers were normalized to week 0 for each clone in L-glutamine-free media.
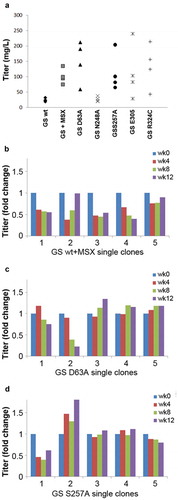
Figure 6. Growth and productivity assessment of single clones derived from GSwt + MSX and GS (R324C) selection in batch culture analysis. The stable pools were generated from CHO-GS−/- clone 2. Growth curves of 4 and 5 randomly selected single clones isolated from GSwt + MSX (a) and R324C (b) stable pools, respectively, are shown. Viable cell density (VCD) was measured every day. The antibody GA101 titer of clones derived from GSwt + MSX (c) and R324C (d) stable pools were measured using nephelometer every other day. Growth and productivity were assessed until the viability dropped below 50%. Antibody GA101 titer measurement of top 20 producing clones from GSwt + MSX (e) and R324C (f) stable pools were assessed in a 14-day batch culture. Stability assessment was done for the single clones with productivity more than 150 mg/L of the antibody isolated from GSwt + MSX (g) and R324C (h) stable pools. The single clones were seeded at 3x103/ml in glutamine-free 50/50 medium for a 14-day batch culture analysis of antibody GA101 production stability at week 0, 4, 8, and 12. Titers were analyzed via nephelometer.
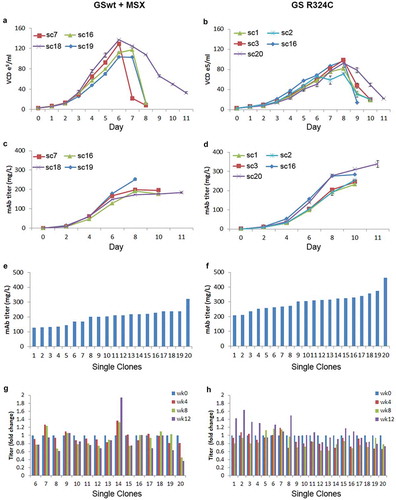
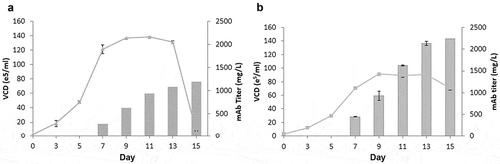
Figure 7. Fed-batch analysis of top producing stable clone derived from GSwt + MSX and GS (R324C) selection. The top producing and stable single clones isolated from GSwt + MSX (a) and R324C (b) stable pools derived from CHO-GS−/- clone 2 were seeded in glutamine-free 50/50 medium. Cells were fed with EX-CELL Advanced Feed 1 at 7.5% (v/v) at Day 3, 5, 7, 9 and 11. Glucose levels were maintained at above 4 g/L. Viable cell numbers, viability, and antibody titers were assessed at the time points indicated in the graph, and fed-batch cultures were terminated when the viability dropped below 50%.