Figures & data
Figure 1. Relative range of potency for some of the more commonly used ADC payloads against human cancer cell line in vitro (adapted from Nakada et al.Citation60).
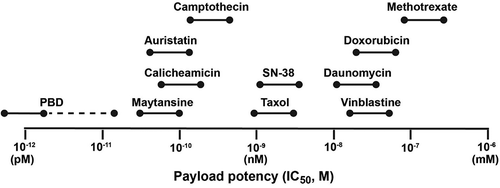
Figure 2. Sacituzumab govitecan (IMMU-132) composition. Sacituzumab govitecan is composed of the humanized monoclonal IgG (designated hRS7) that binds to TROP-2. The IgG is mildly reduced to expose 8 sulfhydryl-binding sites that are subsequently coupled to the CL2A-SN-38 linker-drug through the maleimide moiety of the CL2 linker. As indicated, the CL2A linker has a short PEG (polyethylene glycol) residue to aid in solubility and is coupled to SN-38 at the 20th position of the lactone ring, which stabilizes the ring from opening to the less active carboxylate form. The bond between CL2A and SN-38 is pH sensitive, being more susceptible to release in a low pH environment (e.g., found in lysosome or even in the microenvironment of tumors). The 10th position of SN-38 is protected from glucuronidation while SN-38 is bound to the IgG. Thus, SN-38, while bound to the antibody, remains in its most potent form until released.
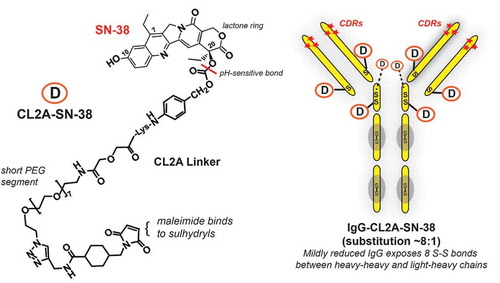
Table 1. Summary of published results on phase 2 trials with sacituzumab govitecan.
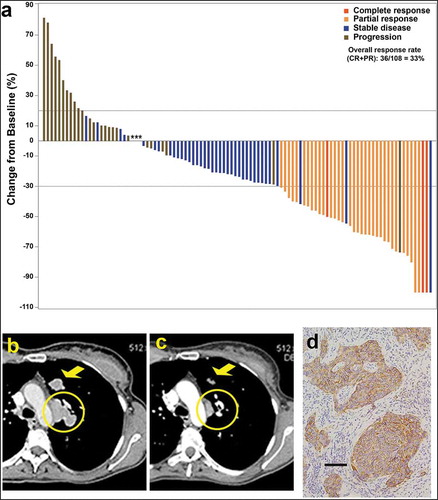
Figure 3. (a) Waterfall plot illustrating the best response data in 108 triple-negative breast cancer patients treated with sacituzumab govitecan (adapted from Bardia et al.,Citation50 with permission). (b–d) Tumor shrinkage by CT in triple-negative breast cancer patient given sacituzumab govitecan (from Bardia et al.Citation38 with permission). Case study: A 48-year-old woman with an initial diagnosis of triple-negative breast cancer in received four prior lines of treatment (including an anti-PD-L1 immune checkpoint inhibitor) and presented with lung and lymph node metastases at enrollment. She achieved a partial response that started 1.7 months after initiation of treatment with sacituzumab govitecan, with the best response of 54% reduction at 9.0 months and progression occurring at 14.4 months (partial response duration, 12.7 months). (b) Baseline image of two of the three target lesions: a 24 x 19-mm left-upper-lung mass (arrow) and a mediastinal lymph node (17 x 29-mm; circle). (c) After 16 doses, these two target lesions decreased to 13 × 7 and 9 × 19 mm, respectively. (d) TROP-2 expression in an archived tumor specimen by immunohistochemistry that shows 1+ to 2+ staining (overall, 2+).