Figures & data
Figure 1. Composition of IgG2/4 control antibody NHDL
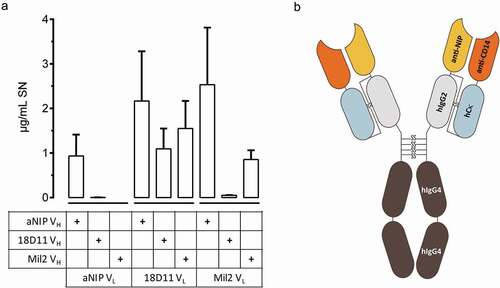
Figure 2. Structural integrity of NHDL compared to other IgG2/4 antibodies
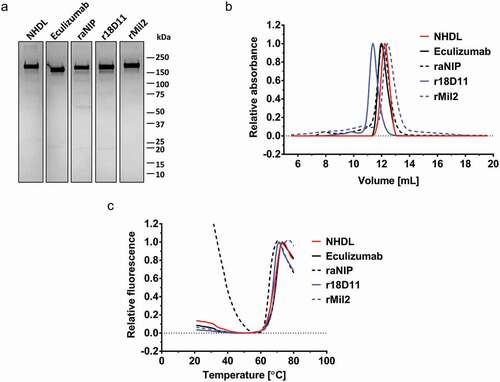
Figure 3. Test of binding and blocking activity of human CD14 by NHDL
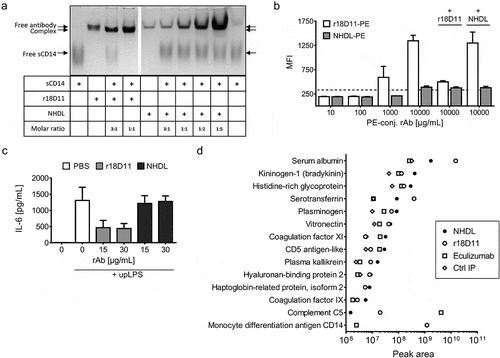
Figure 4. Test of binding and blocking of human C5 and porcine CD14 by NHDL
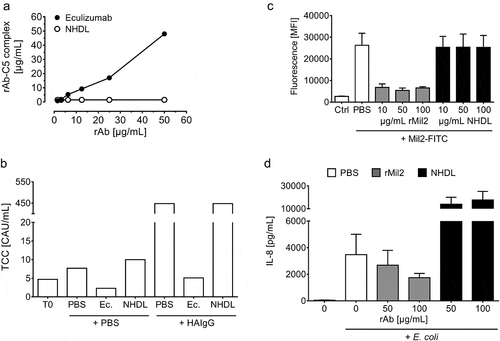
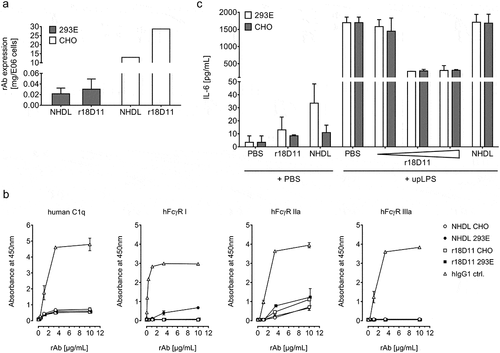
Figure 5. Characterization of CHO-produced NHDL and r18D11