Figures & data
Figure 1. HX008 efficiently blocks the interaction of PD-1 with PD-L1 and PD-L2. (a) HX008 and nivolumab both inhibit PD-L1 from binding to PD-1 in a competition ELISA. Binding of 0.3 μg/mL PD-L1-mFc to plate-coated PD-1-hFc in the presence of increasing concentrations (4.12 ~ 3000 ng/mL) of HX008 or nivolumab. (b) Binding of 1 μg/mL human PD-L2 to plate-coated human PD-1 was inhibited by serially diluted HX008 or nivolumab (4.12 ~ 3000 ng/mL). Results were shown as mean ± SD of one representative experiment out of three
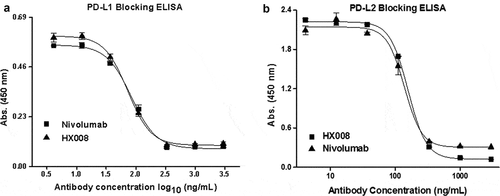
Figure 2. Concentration-dependent stimulation of IL-2 and IFNγ secretion induced by HX008 in mixed lymphocyte reaction (MLR) assays, using nivolumab and human IgG4 as positive and negative controls, respectively. T cells and DCs from different donors were co-cultured in the presence of a titration of anti-PD-1 mAbs or isotype control antibodies, then the level of IL-2 and IFNγ in supernatant was determined after 5 days. (a) enhancement of IL-2 secretion by anti-PD-1 mAbs. (b) enhancement of IFNγ secretion by anti-PD-1 mAbs. Representative data from multiple DC/T cell pairs of different donors were shown
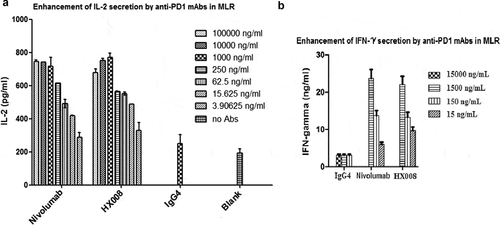
Figure 3. Biological activity evaluation of HX008 in a cell-based luciferase reporter assay. CHO-PD-L1-CD3L cells and the Jurkat-PD-1-NFAT cells were seeded into plates at a ratio of 1:2. After 6 h of incubation, levels of luciferase activity were determined in the presence of serially diluted anti-PD-1 antibodies. All the points in the figures (from one representative data out of three experiments) are average of triplicate with standard deviation
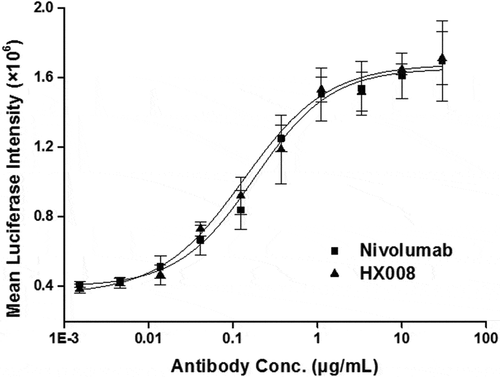
Figure 4. Antitumor response of HX008 in Mixeno model. (a) tumor growth curves of HCC827 tumors in NSG mice. Human PBMC were intravenously (i.v.) injected into mice prior to a subcutaneous HCC827 inoculation. The mice were treated with HX008 (10 mg/kg, or 5 mg/kg; n = 8/group) or nivolumab (5 mg/kg, n = 8) or isotype control antibody (5 mg/kg, n = 8) on Day 6, 9, 13, 16, 19 and 22 after inoculation, respectively. Each group had eight mice, and tumor volume was measured twice weekly. (b) the results of the body weight changes in the tumor-bearing mice. (c) group mean tumor volume measured on Day 9. (d) group mean tumor volume measured on Day 13. For comparisons between groups in the study, we used one-way ANOVA with the Dunn-Sidak post hoc test. A P value of <0.05 was considered to be statistically significant (compared with isotype control). Data were shown as SEM of 8 mice per group; *, P < .05; **, P < .01
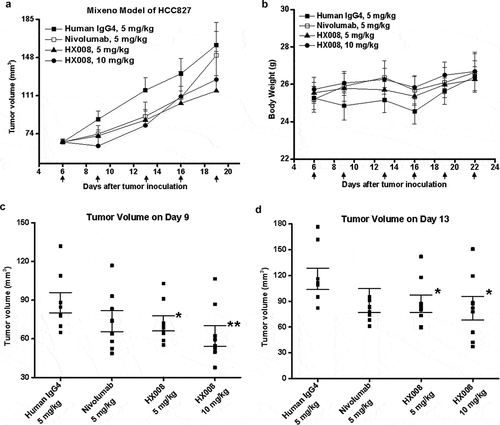
Figure 5. Antitumor response of HX008 in HuGEMM Model. Human PD-1 knock-in mice were subcutaneously implanted with MC38 cells (1 × 106 per mouse) and were randomized into treatment groups after mean tumor volume reached 134 mm3. Single i.v. administration of HX008 (5 mg/kg or 10 mg/kg, n = 8/group) or positive control pembrolizumab (10 mg/kg, n = 8) and isotype control (10 mg/kg, n = 8) were performed twice weekly as indicated, six times in total. Tumor volume was measured twice a week and mice were euthanized at maximum allowed tumor burden. (a) tumor growth curves. (b) group mean tumor volume measured on Day 13 post dose. (c) individual tumor growth curves in each treatment group. The number of total free mice per group was indicated. (d) Body weight of HuGEMM MC38 bearing mice during the treatment. For comparisons between groups in the study, we used one-way ANOVA with the Dunn-Sidak post hoc test. Data were shown as SEM of 8 mice per group; ***, P < .001 (compared with control)
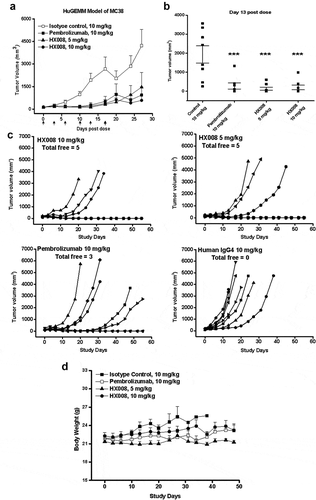
Table 1. Serum PK parameter estimates for HX008 following single i.v. administration to cynomolgus monkey
Figure 6. Mean (± SD) serum concentration-time profiles following a single i.v. administration of 1, 3, 10 mg/kg HX008 and 10 mg/kg nivolumab to cynomolgus monkeys. N = 8 monkeys per time point per group. Serum concentrations were determined using a validated antigen capture ELISA for the two mAb. PK data were shown without effect of anti-drug antibodies (ADA)
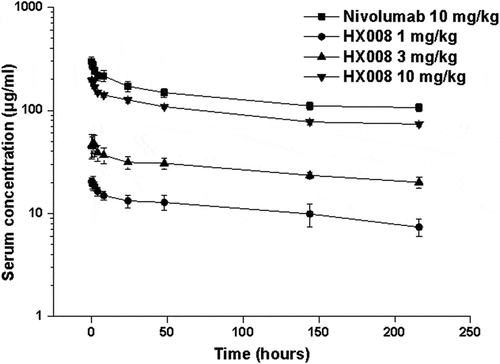
Table 2. Summary statistics for PK parameters of HX008 in patients with advanced solid tumors
