Figures & data
Figure 1. Generation of anti-MSLN antibodies targeting either membrane-proximal or distal regions within MSLN. (a) Comparative binding curves of anti-MSLN mAb#1, mAb#2, and amatuximab. NCI-N87 cancer cells were incubated with a serial dilution of AF488-labeled mAbs for 30 min at 4°C before the AF488 signal was analyzed on a flow cytometer as mean fluorescence intensity (MFI) per condition. EC50 values (in ng/mL) were calculated by the Prism 5 software. A representative graph of three independent experiments is shown. (b-c) Competitive binding assay of anti-MSLN mAbs using a cell-based fluorescent assay format. AF488-labeled 22A10 mAb (b) or amatuximab (c) tested at 10 μg/mL antibody was incubated with either a serially dilution (7.5 ng/mL – 2 mg/mL) of anti-MSLN mAb#1, mAb#2 or an irrelevant hIgG1 isotype control used as competitor antibodies. The mixtures were incubated with NCI-N87 cells for 10 min at 4°C. AF488 staining was analyzed by flow cytometry. Data represent the mean values ± SEM of a minimum of two independent experiments. (d) A protein structure model of human MSLN depicting the binding domains of mAb#1 and mAb#2
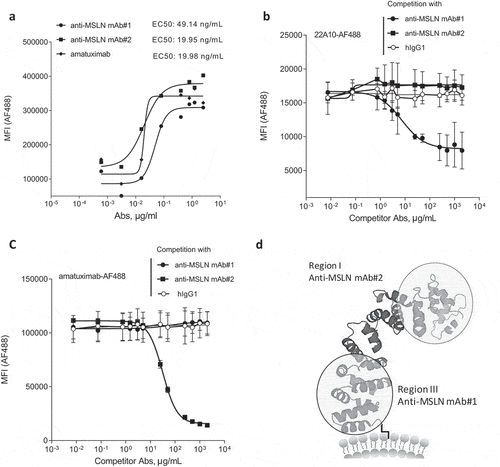
Figure 2. The anti-MSLN Ab targeting a membrane-proximal region showed more efficient killing through ADCC but not by ADCP as compared to Abs targeting more membrane-distal region. (a) %ADCC mediated by a fixed dose of hIgG1 isotype control (e.g., 1 μg/mL) or a dose range (0.03 ng/mL – 1 μg/mL) of anti-MSLN mAb#1, mAb#2, or amatuximab using NCI-N87 cells as targets. The graph depicts an example of a dose–response curve tested in duplicate. (b) The graph summarizes the mean of the %ADCC ± SEM mediated by anti-MSLN mAbs or hIgG1 isotype control (all tested at 1 μg/mL) of 3 independent experiments using different donors as a source of effector cells. (c) ADCP of NCI-N87 target cells with a fixed concentration of hIgG1 isotype control (e.g., 100 μg/mL) or a dose–response of anti-MSLN mAb#1, mAb#2, or amatuximab (0.01 ng/mL – 100 μg/mL). The graph depicts a representative ADCP curve obtained and tested in triplicate. Data are presented as an index of phagocytosis defined as the number of target cells engulfed per 100 macrophages. (d) The graph depicts the maximum index of phagocytosis (e.g., obtained at 10 μg/mL) ± SEM mediated by anti-MSLN mAbs or hIgG1 isotype control (all tested at 10 μg/mL) of 4 independent experiments using different donors as a source of macrophages. Statistical analysis was performed using the unpaired T-test: **p < .01, ns = not significant
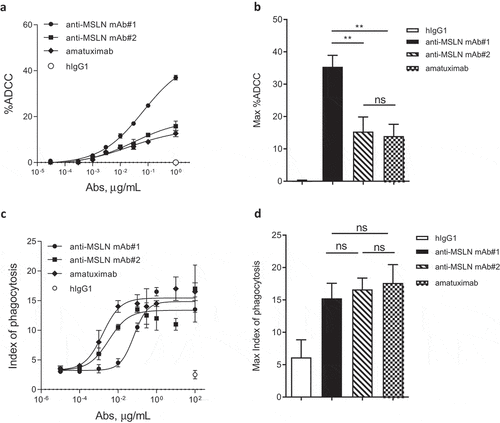
Figure 3. CD47-targeting bsAb engaging a MSLN-membrane proximal region increases the elimination of tumor cells by both ADCC and phagocytosis. Anti-MSLN mAbs generated were converted into a CD47-targeting bsAb format sharing the same CD47 arm (e.g., similar affinity). Anti-CD47xMSLN bsAbs were compared in phagocytosis (a, b) or ADCC (c, d) assays. NCI-N87 cells were incubated with donor-derived macrophages (A, B) or PBMC (C, D) and increasing concentrations of either the CD47xMSLN bsAb#1 or the bsAb#2. The hIgG1 was tested at the maximum concentration (e.g., 100 or 1 μg/mL for phagocytosis and ADCC, respectively). A representative ADCP (a) and ADCC (c) dose–response curve is shown. (b, d) Graphs summarizing maximum killing induced either by the CD47xMSLN bsAb#1, bsAb#2, or the hIgG isotype control, all tested at 100 or 1 μg/mL for phagocytosis (b) and ADCC, respectively (d). Graphs are combining maximum mean killing values by ADCP and ADCC ± SEM of 7 and 3 independent experiments, respectively. Statistical analysis was performed using the unpaired T-test: **p < .01, ***p < .001
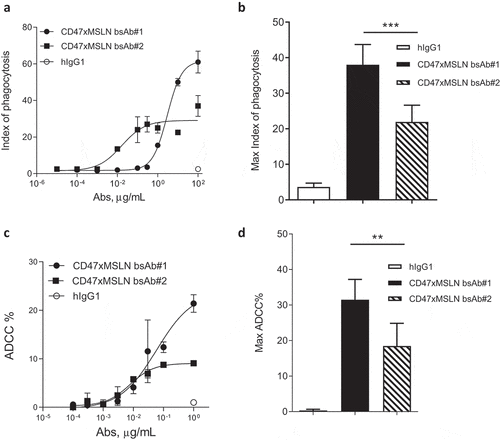
Figure 4. Targeting different membrane-proximal epitopes within MSLN affords CD47-targeting bsAbs similar tumoricidal activity. Binding domain characterization of CD47xMSLN bsAb#1 and bsAb#3 (a–c) using a cell-based fluorescence assay format and NCI-N87 cells as a target. AF488-labeled CD47xMSLN bsAb#1 tested at 10 μg/mL was incubated with a dose–response (7.5 ng/mL – 2 mg/mL) of naked CD47xMSLN bsAb#1, bsAb#3, or an irrelevant isotype control used as competitor antibodies (a). AF488-labeled anti-MSLN mAbs (22A10, 7D9, or amatuximab) tested at 10 μg/mL were incubated with a dose–response of naked CD47xMSLN bsAb#1 (b) or bsAb#3 (c) used as competitors. The mixtures were incubated on NCI-N87 cells for 10 min at 4°C. Resulting fluorescence was analyzed by flow cytometry. Data represent the mean values ± SEM of a minimum of two independent experiments. Comparative in vitro tumoricidal activities by ADCC (d, e) and ADCP (f, g). A representative dose-–response is shown for ADCC (d) and ADCP (f). Maximum killing ADCC (e) and ADCP (g) efficacy mediated by CD47xMSLN bsAb#1 and bsAb#3 are presented, all tested at 1 μg/mL or 100 μg/mL, respectively. Data are means ± SEM of a minimum of five independent experiments. Statistical analysis was performed using the unpaired T-test: ns = not significant
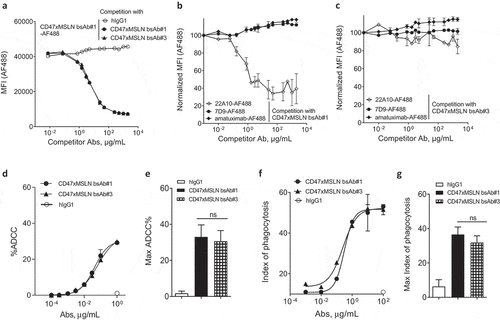
Figure 5. Engaging a membrane-proximal region within MSLN increases FcγR-IIIA signaling. NCI-N87 target cells were incubated with a dose range of either CD47xMSLN bsAb#1 or bsAb#2 (a–c), then mixed with engineered FcγR-I (a), FcγR-IIA (H131 polymorphism) (b) or FcγR-IIIA (V158 polymorphism) (c) reporter Jurkat cell lines. After 6 h of co-culture at 37°C, the luciferase activity was measured. Data are then presented as fold change induction. Graphs depict a representative dose–response curve obtained for a minimum of two independent experiments tested in duplicate
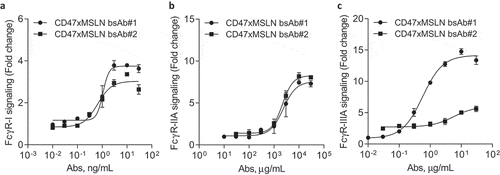
Figure 6. Pairing a membrane-proximal epitope of MSLN to a CD47-blocking arm results in an efficient disruption of the CD47/SIRPα axis and enhances the phagocytosis potential of a MSLNxCD47 bsAb. ADCP dose-–response curve mediated by the CD47xMSLN bsAb#1 (a) or bsAb#2 (b) compared to their respective monovalent variants containing only the CD47 arm or anti-MSLN arm. A representative experiment (among four independent donors used as a source of effector cells) is depicted in the figure. In specific experiments, target cells were incubated in the presence of the indicated full length or F(ab’)2 fragments (tested at 10 μg/mL) (c). The anti-MSLN mAb 7D9 is tested at suboptimal concentration (3 ng/mL) either alone or combined to B6H12, CD47xMSLN bsAb#1 or bsAb#2 F(ab’)2 fragments (tested at 10 μg/ml each). Graph depicts a summary of four independent experiments (C). Statistical analysis was performed using the unpaired T test: *p < .05, **p < .01, ns = not significant
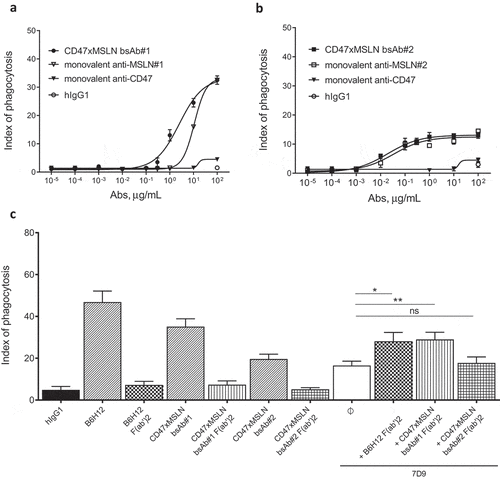
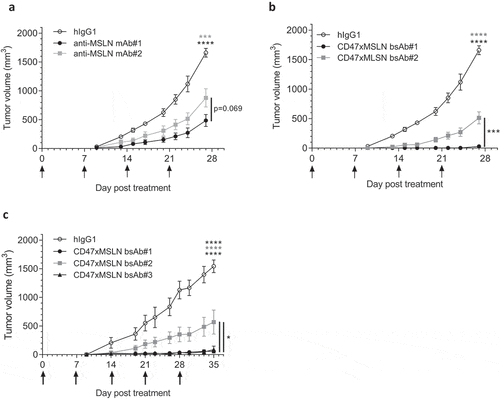
Figure 7. Antitumor activity of anti-MSLN mAbs and CD47xMSLN bsAbs in a xenograft model. HepG2-MSLN-Red-Fluc tumor cells were injected s.c. in the right flank of NOD scid mice. Bioluminescence imaging (BLI) was performed 2 weeks after the graft to measure tumor burden and then mice were subsequently allocated into different groups to obtain similar mean tumor burden between groups. From the next day, antibody treatments were administered once a week i.v. at 6 mg/kg (a, b) or 60 mg/kg (c) starting the day after BLI (D15). Tumor size was measured 3 times a week using a digital caliper and tumor volume determined using the formula (lengthxwidth2) x 0.5. The endpoint of the experiment was fixed at 1500 mm3. Tumor volume is represented as mean ± SEM (n = 7–8 mice/group) and one-way ANOVA with Tukey multiple comparison statistical analysis performed at D27 (A, B) or D35 (C). *p < .05, ***p < .001, ****p < .0001, ns = not significant