Figures & data
Figure 1. Schematic representation of plasmids used to transfect Expi293 cells for the production of benchmark and multivalent anti-TNF Quad proteins. Poly-histidine tag (6xHis) was included in each construct at the C-terminus except for where a second kappa light chain is used in conjunction with a first chain, which contained the his-tag. The * denotes Fc region lacking the core hinge region.
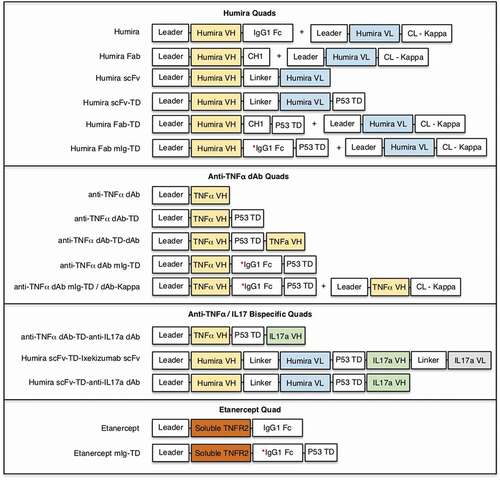
Figure 2. Characterization of secreted multivalent Quad proteins. Soluble expression and purity of the different groups of anti-TNF Quad proteins were demonstrated by SDS-PAGE analysis. Description and size of the protein monomers are indicated (a). The multimeric state and purity of four different Quad formats were further analyzed by size-exclusion chromatography, which included (b) Humira scFv-TD, (c) Humira Fab mIg-TD, (d) anti-TNF dAb-TD-dAb and (e) anti-TNF dAb-TD-IL17a dAb. Proteins eluted from the single main peaks were re-analyzed on SDS-PAGE under reduced conditions to further confirm the authenticity of the Quad proteins. Quad proteins are shown in blue lines and the monovalent control proteins in red.
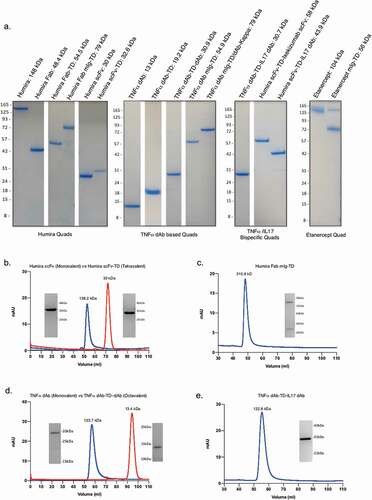
Figure 3. SPR sensograms of Humira, Humira scFv-TD, and Humira scFv to TNF using immobilized Quad proteins (a) or immobilized recombinant TNF protein (b). SAXS analysis developed molecular envelopes and representative models of tetrameric and octameric proteins (Panels C and D). (c) Model of chimeric molecule CD7 scFv-TD (purple) fitted into the experimentally determined scattering envelope. The model suggests the chimeric molecule adopts a cruciform structure in good agreement with the envelope. (d) Model for construct CD20 scFv-TD-scFv (magenta) fitted to an experimentally determined scattering envelope, which again suggests a cruciform structure is adopted by the chimeric molecule.
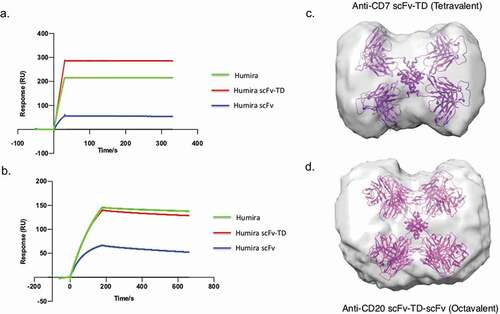
Figure 4. Analysis of antigen-binding properties of anti-TNF Quads. Indirect binding ELISA was used to assess anti-TNF Quad binding to its cognate antigen (TNF). Anti-TNF Quads were analyzed in groups as follows (a) Humira-based Quads (b) anti-TNF dAb Quads without Fc (c) anti-TNF dAb Quads with Fc (d) bispecific anti-TNF/IL17a Quad formats (e). Monospecific anti-TNF dAb vs bispecific anti-TNF dAb and (f) Etanercept-based Quad (n = 2 ± SEM).
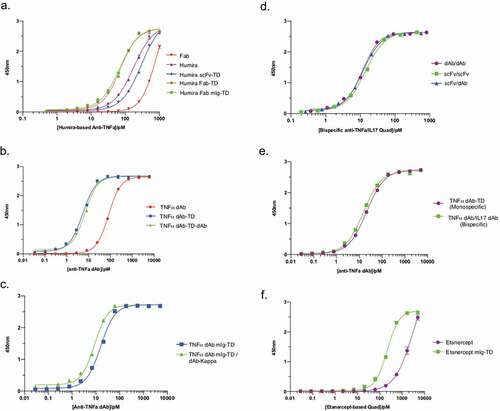
Table 1. A summary of the EC50 values of anti-TNF molecules to neutralize TNF-mediated cytotoxicity in WEHI 164 cells treated with 0.1 ng/ml of human TNF.
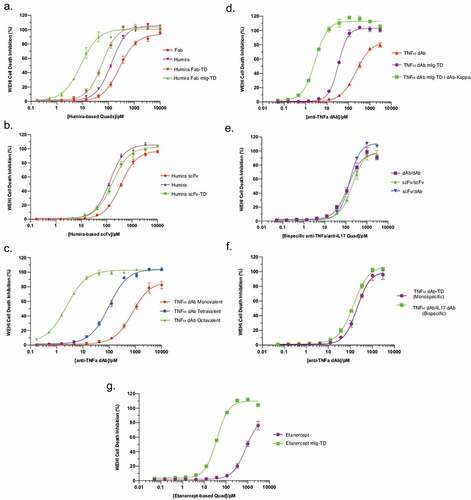
Figure 5. Functional characterization of anti-TNF Quad proteins. Anti-TNF Quad molecules were analyzed for their ability to neutralize TNF-mediated cytotoxicity in WEHI cells. The neutralization curves were plotted in different groups according to their format (n = 3 ± SEM). Benchmark antibodies and monovalent control were also included. The groups included Humira-based Quads (a), Humira-based scFv (b), anti-TNF dAb Quads without Fc (c) anti-TNF dAb Quads with Fc (d), bispecific anti-TNF/IL17a Quad formats (e), monospecific anti-TNF dAb vs bispecific anti-TNF dAb (f) and Etanercept-based Quad (g).