Figures & data
Table 1. General description of the mAbsa
Table 2. Biophysical and FcRn binding properties of the mAbsa
Table 3. Rat pharmacokinetic parameters of the mAbs
Table 4. Cynomolgus monkey pharmacokinetic parameters of the mAbs
Figure 1. Subcutaneous tissue association of the Platform 1 and 3 parent and re-engineered molecules following a single administration of 0.1 mg/kg of each 125I labeled mAb. Data show the relative SC tissue association of each re-engineered mAb relative to their respective parental mAb. The 1-h post dose time point skin punctures radioactive count for each mAb was considered 100% bound for data normalization purposes. The 6-h post dose collected radioactivity data were compared reported as a fraction of the percent bound relative to the 1-h post dose time point for calculation, data processing and loss of mAb from the SC site reporting over time. Data are the average of two independent SC tissue assessment from two cynomolgus monkeys for each mAb.
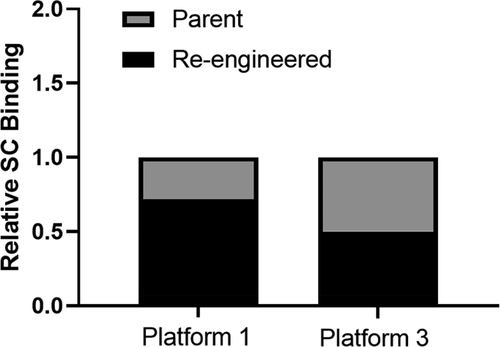
Table 5. Correlation scores for the relationship of the physiochemical parameters with the PK of the mAbs assessed following a single IV administration to ratsa
Table 6. Correlation scores for the relationship of the physiochemical parameters with the PK of the mAbs assessed following a single SC administration to ratsa
Table 7. Correlation scores for the relationship of the physiochemical parameters with the PK of the mAbs assessed following a single IV administration to cynomolgus monkeysa
Table 8. Correlation scores for the relationship of the physiochemical parameters with the PK of the mAbs assessed following a single SC administration to cynomolgus monkeysa
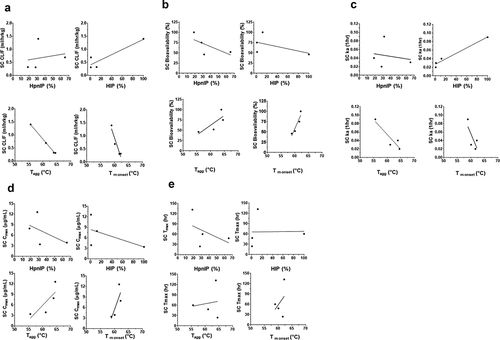
Figure 2. Correlation plots of the rat pharmacokinetic parameters following SC administration of mAbs 1P and 1RE in Platform 1, mAbs 2P and 2RE in Platform 2 and mAbs 3P and 3RE in Platform 3. Correlation plots for the (a) CL/F, (b) SC bioavailability, (c) rate of SC absorption (ka), (d) Cmax and (e) Tmax with the physiochemical properties HpnIP, HIP, Tagg and Tm onset.
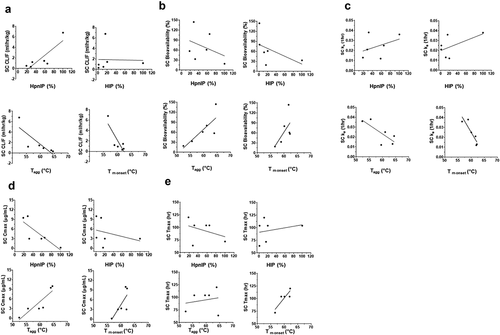
Figure 3. Correlation plots of the cynomolgus monkey pharmacokinetic parameters following SC administration of mAbs 1P and 1RE in Platform 1 and mAbs 3P and 3RE in Platform 3. Correlation plots for the (a) CL/F, (b) SC bioavailability, (c) rate of SC absorption (ka), (d) Cmax and (e) Tmax with the physiochemical properties HpnIP, HIP, Tagg and Tm onset.