Figures & data
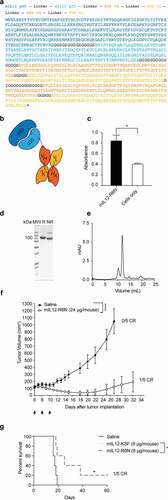
Figure 1. Characterization of antibodies against the human spliced isoform D of Tenascin C (hTNC-D)
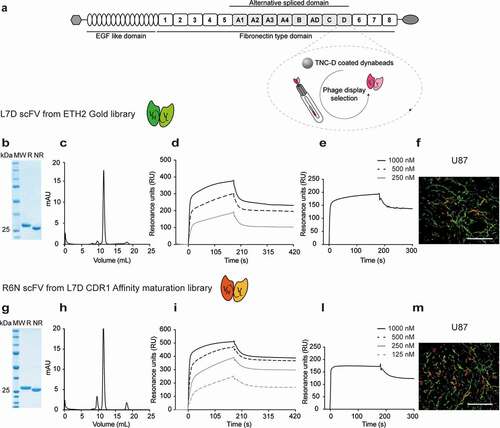
Figure 2. Microscopic fluorescence analysis of TNC-D expression on xenografts and tumors of mouse origin section with R6N IgG1-FITC
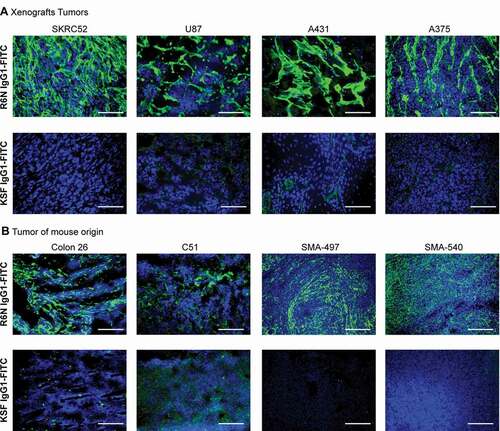
Figure 3. Microscopic fluorescence analysis of TNC-D expression of frozen tumor and normal tissues
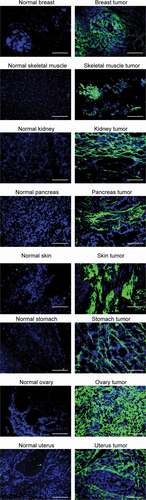
Figure 4. Immunofluorescence-based biodistribution analysis with R6N IgG1
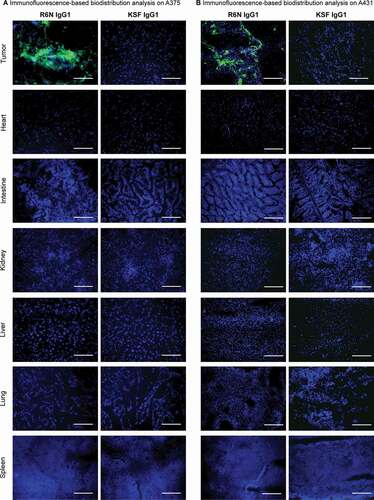
Figure 5. Therapy in BALB/c nude mice bearing SKRC52 human renal cell carcinoma and in VM/Dk mice bearing SMA-497 glioma