Figures & data
Table 1. A comparison of antibody-based therapies for COVID-19
Figure 1. Characterization of antibody-based therapies for SARS-CoV-2
A. In convalescent plasma, the polyclonal immune response is featured by polyclonal IgM, IgA, and IgG. These immunoglobulins can either bind the SARS-CoV-2 spike protein (neutralizing or not), other SARS-CoV-2 antigens or other antigens. B. In hyperimmune serum, the active ingredient is represented by polyclonal IgG, directed either against SARS-CoV-2 (spike or other proteins) or other antigens. C. Anti-SARS-CoV-2 neutralizing mAbs cloned from anti-Spike IgG provide a monoclonal immune response. Ig: immunoglobulins; mAbs: monoclonal antibodies; ACE2: type 2 angiotensin-converting enzyme
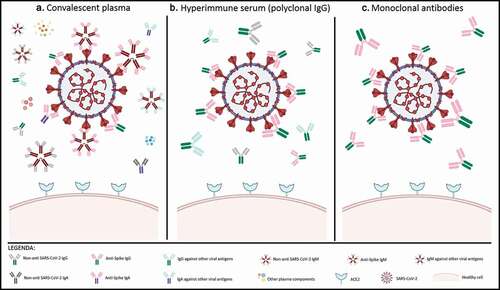
Table 2. mAb-based therapeutics for COVID-19 in clinical trials
