Figures & data
Figure 1. hCXCR2-KI mice design and characterization
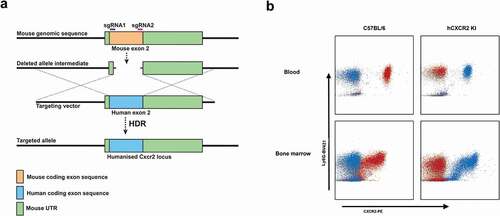
Figure 2. Anti-hCXCR2 mAb characterization
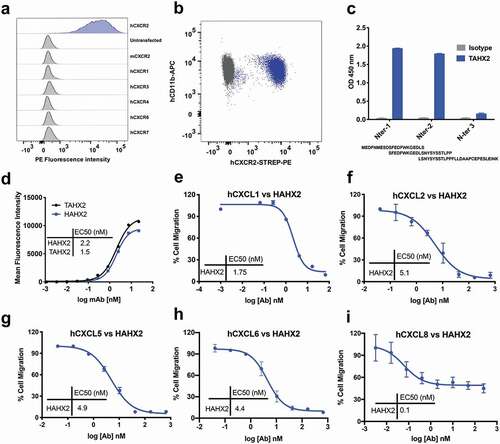
Figure 3. Surrogate anti-mCXCR2 mAb attenuates MC903-induced skin inflammation. C57BL/6 WT mice ears were topically administered with 1 nmol MC903 or EtOH (as vehicle control) for 14 days. On day 5, when ears exhibited symptoms of AD-like skin inflammation, a loading dose of SAMX2-FcKO or isotype control mAb at 20 mg/kg of body weight was injected i.p., followed by 5 mg/kg every other day for 1 week. (a) Schematic of the treatment regimen (b) Representative appearance of EtOH, MC903, and SAMX2-FcKO mAb-treated ears at the endpoint. (c) Ear thickness was measured at each treatment day. (d) Representative ear sections stained with H&E. Original magnification = × 20, scale bar = 200 μm. (e) Ear itch was assessed at day 14 (24 hours prior the endpoint). (f) Epidermis thickness (Acanthosis) and (g) Dermal thickening were measured in tissue sections and quantified by ImageJ software (National Institutes of Health). Each data point represents an individual ear or mouse, and n = 6 mice in each group. All data represented as mean ± standard error of the mean. Each graph is representative of three independent experiments. Ear thickening was analyzed with the two-way analysis of variance with Tukey posttest. Itch assessment and histological measurement was analyzed with the one-way analysis of variance with Tukey posttest. *P < .05, **P < .01, ***P < .005, ****P < .0001
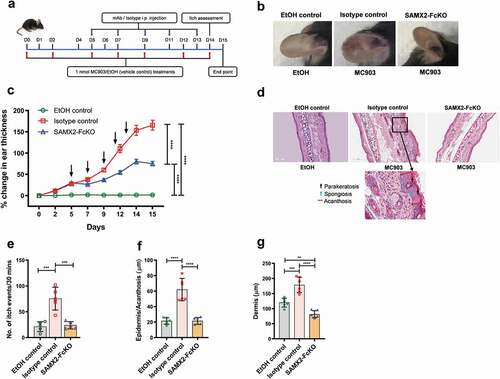
Figure 4. Humanized anti-hCXCR2 mAb suppress MC903-induced skin inflammation in human CXCR2 Knock-in mice. hCXCR2-KI mice ears were topically administered with 1 nmol MC903 or EtOH (as vehicle control) for 14 days. On day 9, when ears exhibited symptoms of AD-like skin inflammation, a loading dose of HAHX2-FcKO or isotype control mAb at 20 mg/kg of body weight was injected i.p., followed by 5 mg/kg every other day for 1 week. (a) Schematic of the treatment regimen (b) Representative appearance of EtOH, MC903, and HAHX2-FcKO mAb-treated ears at the endpoint. (c) Ear thickness was measured at each treatment day. (d) Representative ear sections stained with H&E. Original magnification = × 20, scale bar = 200 μm. (e) Ear itch was assessed at day 14 (24 hours prior the endpoint). (f) Epidermis thickness (Acanthosis) and (g) Dermal thickening were measured in tissue sections and quantified by ImageJ software (National Institutes of Health). Each data point represents an individual ear or mouse, and n = 6 mice in each group. All data represented as mean ± standard error of the mean. Each graph is representative of three independent experiments. Ear thickening was analyzed with the two-way analysis of variance with Tukey posttest. Itch assessment and histological measurement was analyzed with the one-way analysis of variance with Tukey posttest. *P < .05, **P < .01, ***P < .005, ****P < .0001
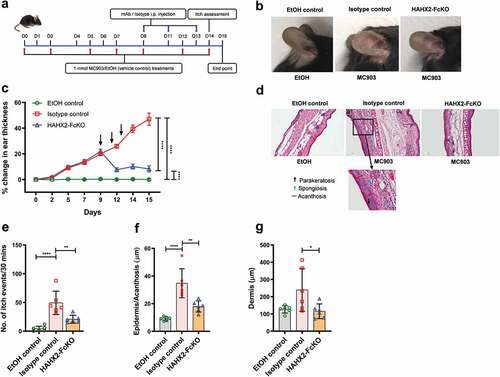
Figure 5. Anti-CXCR2 mAbs treatment reduces leukocytes infiltration in inflamed ear skin and ear draining lymph nodes. C57BL/6 WT and hCXCR2-KI mice were topically administered with 1 nmol MC903 or EtOH (vehicle control) for 14 days. When mice ear exhibiting symptoms of AD-like skin inflammation (on day 5 for WT, and day 9 for hCXCR2-KI mice), a loading dose of SAMX2-FcKO, HAHX2-FcKO, or isotype control mAb at 20 mg/kg of body weight was injected i.p., followed by 5 mg/kg every other day for 1 week. At the end of the experiment, single-cell preparation from ear tissues and ear draining lymph nodes (dLNs) were prepared and analyzed by flow cytometry. The effect of SAMX2-FcKO or HAHX2-FcKO mAb treatment on the infiltration of: (a) Myeloid cells in ear skin of C57BL/6 WT mice, (b) Lymphoid cells in ear skin of C57BL/6 WT mice, (c) Myeloid cells in ear skin of hCXCR2-KI mice, (d) Lymphoid cells in ear skin of hCXCR2-KI mice, (e) Myeloid cells in ear dLNs of C57BL/6 WT mice, (f) Lymphoid cells in ear dLNs of C57BL/6 WT mice, (g) Myeloid cells in ear dLNs of hCXCR2-KI mice, (h) Lymphoid cells in ear dLNs of hCXCR2-KI mice, at the endpoint. Each data point represents an individual mouse, and n = 6 in each group. All data represented as mean ± SEM. Each graph is representative of three independent experiments. Statistics were calculated using one-way analysis of variance with Tukey posttest. *P < .05, **P < .01, ***P < .005, ****P < .0001
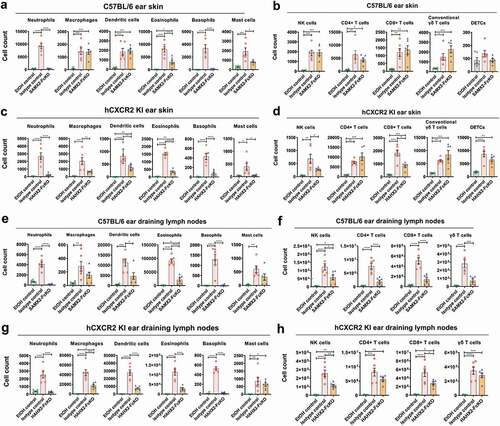
Figure 6. Surrogate anti-mCXCR2 mAb reverse the K/BXN-serum transfer arthritis. C57BL/6 WT mice were i.p. injected 200uL of K/BXN serum on day 0 and 1. The development of arthritis was assessed by measuring the ankle thickness and clinical index score every day until the experimental endpoint. When mice exhibiting symptoms of arthritis and cumulative clinical score reached at 4 (on day 4), mice split into two groups, injected i.p., SAMX2-FcKO or isotype control mAb at 20 mg/kg of body weight, followed by 5 mg/kg every other day for 1 week (a) Scheme of the treatment regimen. (b) Representative images of ankle of mice at the endpoint. (c) Clinical score. (d) Ankle thickness. (e) and (f) Histological examination of the representative ankle joint stained with H&E (e), and Safranin O (f). Mice that did not receive K/BXN serum or antibody treatment were include as a control group (normal control). Data are representative of three independent experiments with 6 animals per group. All data represented as mean ± standard error of the mean. Statistics were calculated using two-way analysis of variance with Tukey posttest. ***p < .005, ****p < .0001
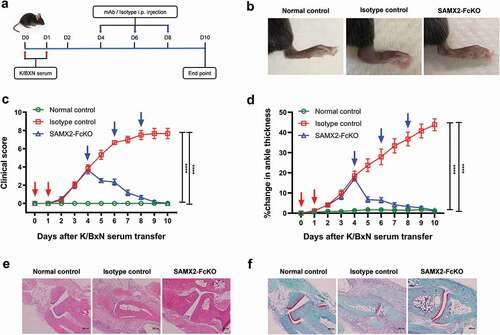
Figure 7. Humanized anti-hCXCR2 mAb reverse arthritis in human CXCR2 Knock-in mice. hCXCR2-KI mice were i.p. injected 200uL of K/BXN serum on day 0 and 1. The development of arthritis was assessed by measuring the ankle thickness and clinical index score every day until the experimental endpoint. When mice exhibiting symptoms of arthritis and cumulative clinical score reached at 4 (on day 4), mice split into two groups, injected i.p., HAHX2-FcKO or isotype control mAb antibody at 20 mg/kg of body weight, followed by 5 mg/kg every other day for 1 week (a) Scheme of the treatment regimen. (b) Representative images of ankle of mice at the endpoint. (c) Clinical score. (d) Ankle thickness. (e) and (f) Histological examination of the representative ankle joint stained with Hematoxylin and eosin (e), and Safranin O (f). Data are representative of three independent experiments with 6 animals per group. Mice that did not receive K/BXN serum or antibody treatment were include as a control group (normal control). All data represented as mean ± standard error of the mean. Statistics were calculated using two-way analysis of variance with Tukey posttest. ***p < .005, ****p < .0001
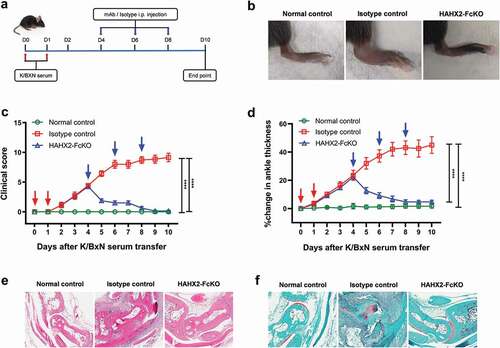
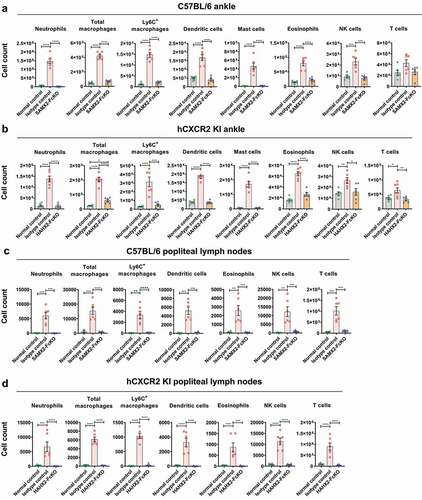
Figure 8. Anti-CXCR2 mAbs treatment reduces leukocytes infiltration to the inflamed ankle and popliteal draining lymph nodes. C57BL/6 WT or hCXCR2 -KI mice were injected i.p. 200uL of K/BXN serum on day 0 and 1. When mice were exhibiting symptoms of arthritis and the cumulative clinical score reached 4 (on day 4), mice split into two groups and injected i.p. SAMX2-FcKO, HAHX2-FcKO, or isotype control mAb at a concentration of 20 mg/kg body weight, followed by 5 mg/kg every other day for 1 week. Flow cytometric analysis demonstrates the effect of SAMX2-FcKO and HAHX2-FcKO mAb treatment on the innate effector cells compare to a control group that did not receive K/BXN serum or antibody treatment (normal control): (a) C57BL/6 WT mice ankle and (b) hCXCR2-KI mice ankle, (c) C57BL/6 WT mice draining lymph nodes and (d) hCXCR2-KI mice draining lymph nodes, at the endpoint. Each data point represents an individual mouse, and n = 6 in each group. All data represented as mean ± SEM. Each graph is representative of three independent experiments. Statistics were calculated using one-way analysis of variance with Tukey posttest. *P < .05, **P < .01, ***P < .005, ****P < .0001