Figures & data
Table 1. A summary of pharmacokinetic and other characteristics of typical therapeutic monoclonal antibodies (mAbs), and key differences with small-molecule drugsCitation14,Citation32,Citation33,Citation35,Citation47–49.
Table 2. Preclinical species commonly used in pharmacokinetic translation to human with their typical body weights, brain weights and maximum life potential (MLP)Citation53–55.
Table 3. An overview of the commonly used allometric scaling approaches for monoclonal antibodies (mAbs) with the typical values of the allometric weight exponents for clearance
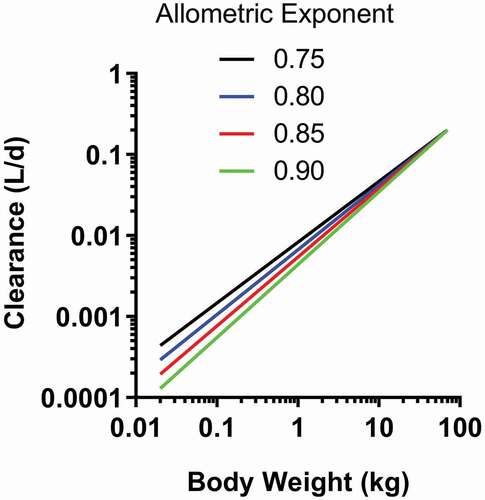
Figure 1. Schematic representation of a 2-compartment population PK model with a full target-mediated drug disposition model for an intravenously (i.v.) administered monoclonal antibody (mAb). CL and Q are central and inter-compartmental clearances, V1 and V2 are central and peripheral volumes, respectively, Ksyn is target production rate constant, Kdeg is target degradation rate constant, Kon is association/binding rate constant, Koff is dissociation rate constant, and Kint is mAb-target complex internalization rate constant. Adapted from.Citation96
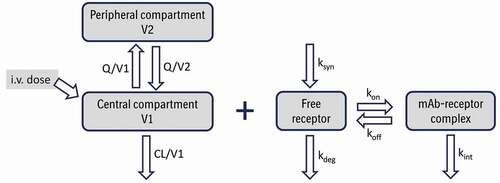
Figure 2. Schematic representation of a 2-compartment population PK model with nonlinear elimination described by Michaelis-Menten approximation for an intravenously (i.v.) administered monoclonal antibody. CL and Q are central and inter-compartmental clearances, V1 and V2 are central and peripheral volumes, respectively, Vmax is the maximum rate of nonlinear elimination and Km the Michaelis-Menten constant; C1 is concentration in the central compartment.Citation96,Citation97 Adapted from Wang et al.Citation14
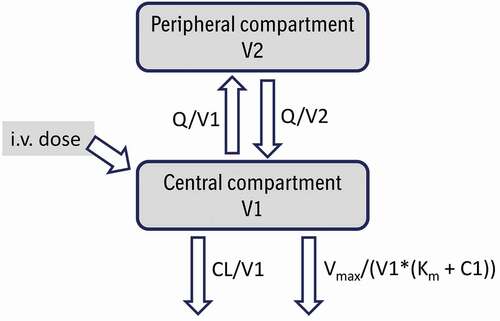
Figure 3. Relationship between clearance and body weight for allometric exponents ranging from 0.75 to 0.90. Human clearance and body weight were fixed to 0.2 L/day and 70 kg, respectively