Figures & data
Figure 1. Generic MAM workflow for biotherapeutic modalities that enables targeted attribute quantitation and new peak detection.
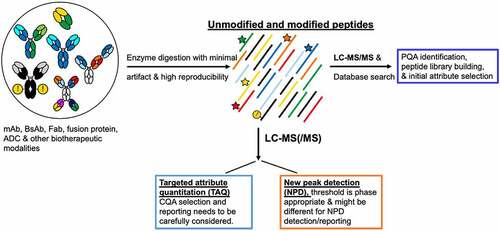
Table 1. General product quality attributes for monitoring or control by conventional methods and by the standard MAM.
Table 2. Considerations of MAM trypsin digestion for minimized artifacts.
Table 3. Considerations of SST controls of bracketing reference standards for MAM targeted variant analysis*.
Table 4. Mean value, standard deviation (SD), and % relative standard deviation (RSD) of % abundance of a subset of representative attributes for mAb4 from the intermediate precision study (n = 24, using three instruments, three column resin lots, with six independent sample preparations by two analysts on two different days).
Figure 2. Comparison between acidic variants measured by MAM and IEC in stability and stress samples of mAb4.
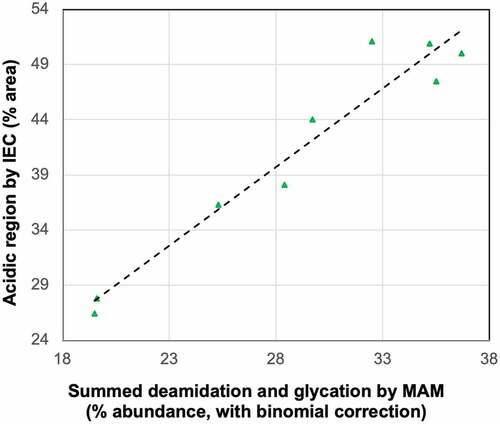
Table 5. Current examples and potential MS applications in QC beyond the peptide mapping-based MAM.
