Figures & data
Figure 1. Number of product approvals each year among HCAPs between 1998–2021. (n = 46).
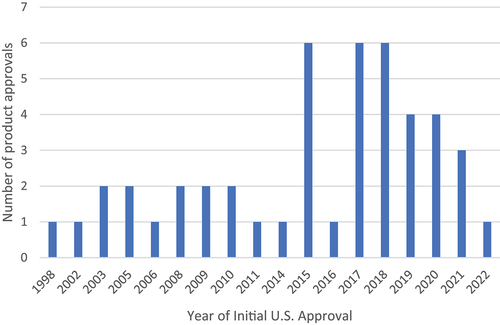
Figure 2. Routes of administration among approved HCAPs (n = 46).
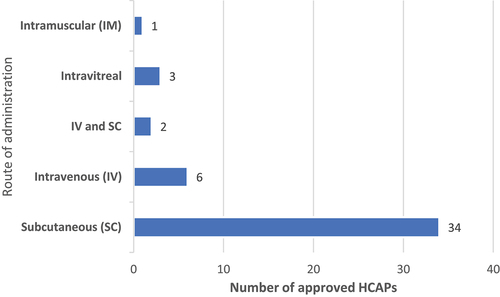
Figure 3. Different therapeutic areas for which HCAPs are approved and marketed (n = 46).
Figure 4. Antibody isotypes and subtypes amongst approved HCAPs.
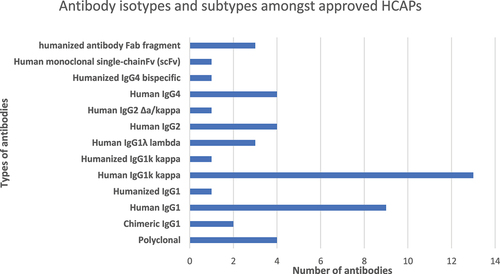
Figure 5. Dosage forms of the approved HCAPs (n = 46).
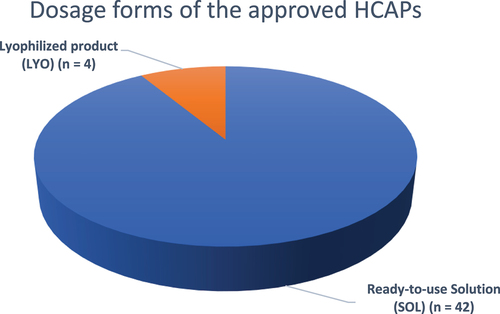
Table 1. List of excipients generally used in protein formulations and their functions.
Figure 6. Tonicity agents utilized in formulation of approved HCAPs (n = 46).
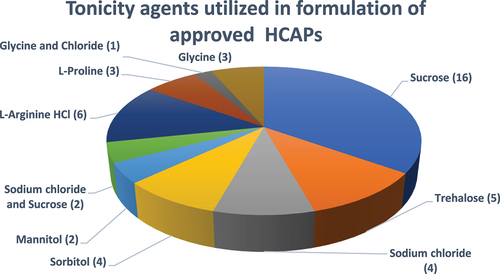
Figure 7. Disaccharide sugars and polyols utilized in formulation of approved HCAPs (n = 46).
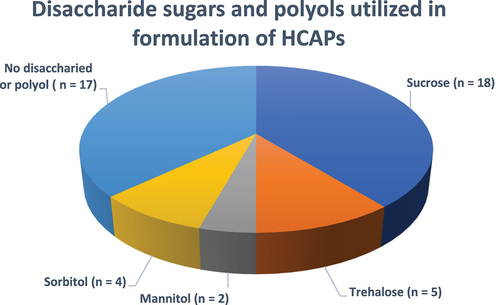
Figure 8. Buffers utilized in formulations of approved HCAPs (n = 46).
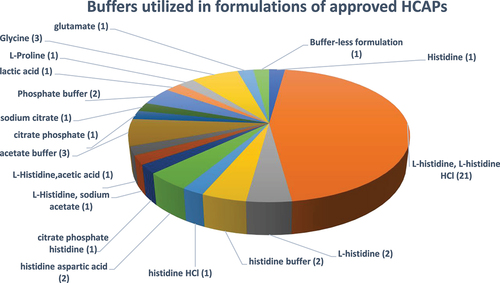
Figure 9. pH of stabilized formulations amongst approved HCAPs (n = 46).
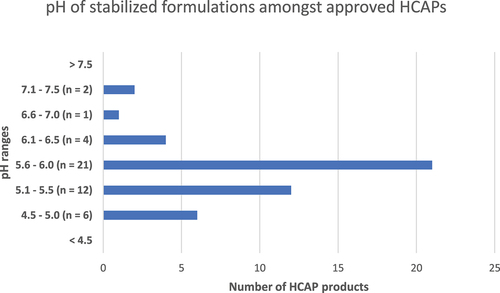
Figure 10. Surfactants in formulations of approved HCAPs (n = 46).
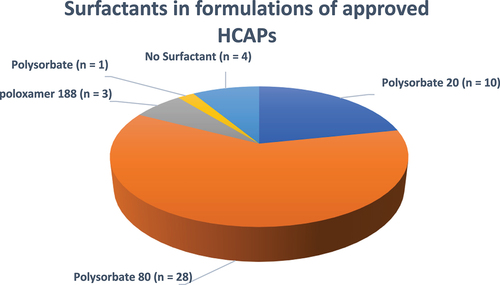
Figure 11. Amino acids utilized in formulations of approved HCAPs (n = 46).
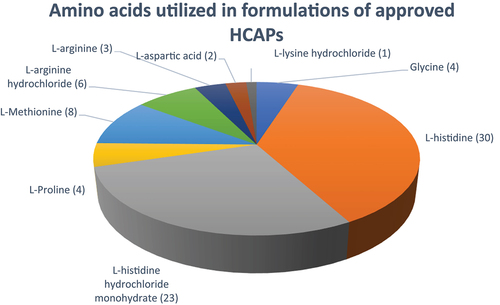
Figure 12. Number of amino acids used in each formulation for stabilization of HCAPs (n = 46).
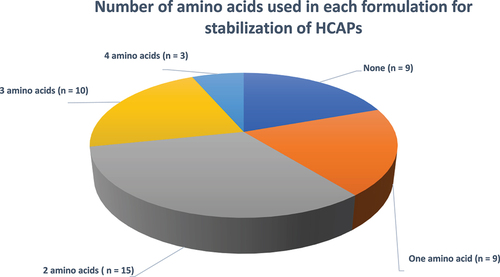
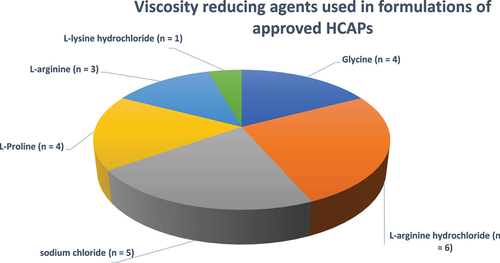
Figure 13. Viscosity reducing agents used in formulations of approved HCAPs (n = 23).