Figures & data
Figure 1. Avidity-driven binding of ACE2 decoys to SARS-CoV-2 Wuhan (WT) strain spike RBD increases with higher ACE2 valency.
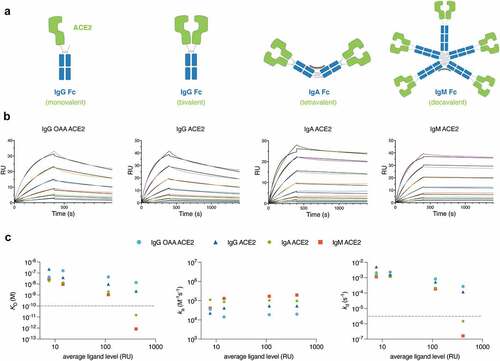
Table 1. Binding kinetics of ACE2 decoy variants and clinical comparators to recombinant spike protein RBD as measured by SPR.
Figure 2. Neutralization of SARS-CoV-2 variant spike protein and ACE2 binding by ACE2 decoys and REGEN-COV mAb cocktail by blocking immunoassay, pseudovirus, and live virus neutralization assays.
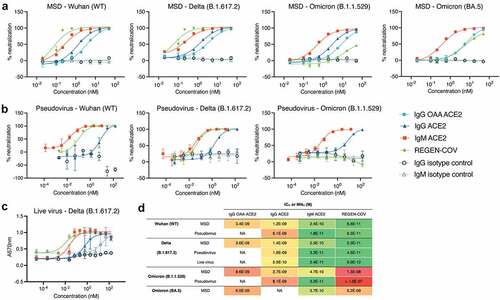
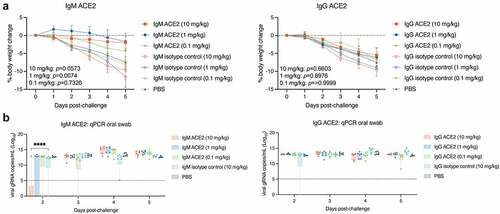
Figure 3. In vivo efficacy of intranasal IgM ACE2 decoy and IgG ACE2 decoy for treatment of SARS-CoV-2 Delta (B.1.617.2) infection in a Syrian hamster model.