Figures & data
Figure 1. Example of standard workflow from gene to final clone.
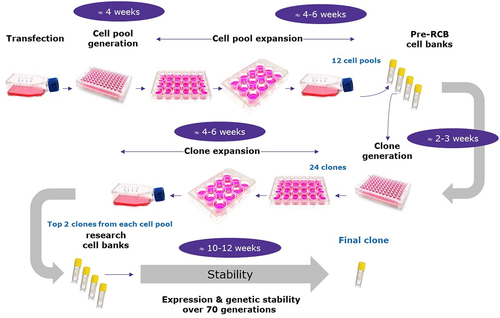
Figure 2. Traditional and accelerated development timelines.
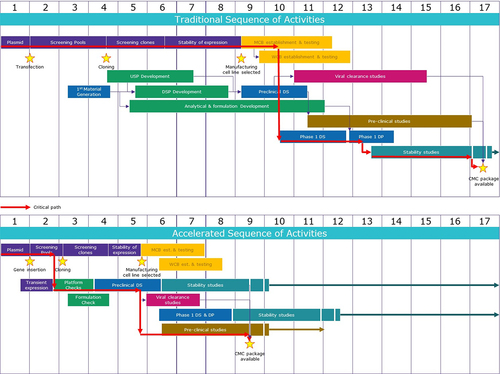
Table 1. Acceleration options to CMC development (from gene to First-in-Human) (text in bold relates to acceleration options for traditional programs whereas text in italic reflects additional pathways potentially applicable to accelerated programs only).
Table 2. Approved COVID-19 monoclonal antibodies.
