Figures & data
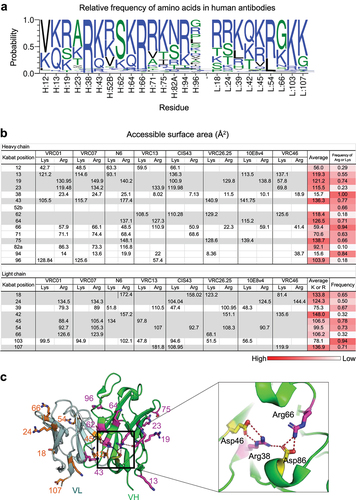
Figure 1. VRC07–523-03FR3 variants with reduced net positive charge showed reduced affinity to heparin and reduced polyreactivity. (a) Closeup view of 03FR3 loop insertion into VRC01.23LS heavy chain. Four Asp’s were highlighted in red. (b) Insertion of 03FR3 loop into VRC07-523LS reduced HEp−2 cell binding. Antibodies scored greater than 1 at 25 µg/ml were considered polyreactive. (c) Sequence alignments of VRC07-523LS variants (d) Polyreactivity of VRC07-523LS variants assessed in HEp−2 cell bindings (left panel). Chromatograms of VRC07-523LS variants on heparin affinity chromatography (right panel). Redlines represent the NaCl gradient. Retention volumes of VRC07-523LS variants on heparin chromatography correlated with polyreactivity. (e) Correlations of heparin retention volume vs. net positive charge, HEp−2 cell bindings vs. isoelectric point (pI), HEp−2 cell bindings vs. net positive charge of VRC07-523LS variants.
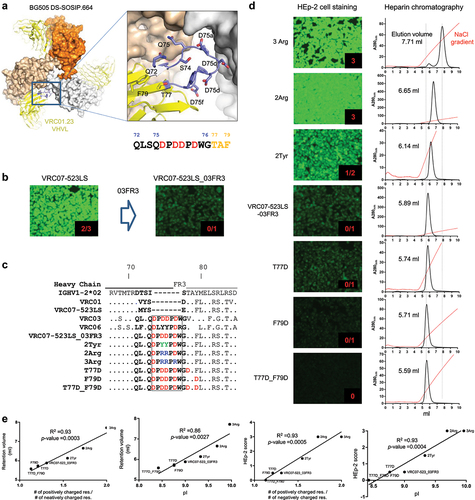
Figure 2. Workflow for identifying antibody variants with improved pharmacokinetics by surface charge alteration. (1) Selection of Arg or Lys residues in the framework region based on accessible surface areas. (2) Screening the residues that contact with the epitope or within 5Å from the epitope. (3) Generation of antibody variants.Selection of candidate variants for PK assessment based on neutralizing potency and breadth, polyreactivity, and retention volumes on heparin affinity chromatography. (5) Assessment of pharmacokinetics in human FcRn knock-in mouse model.
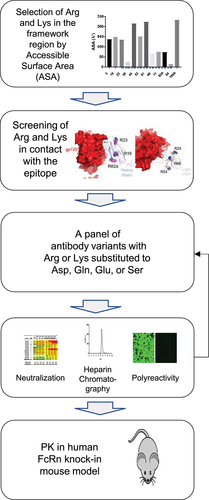
Figure 3. (a) Arg and Lys residues in the variable region of VRC07-523 selected for Asp, Glu, or Sersubstitution were demonstrated on the VRC07-523 Fab modeled using the VRC01.23-bound BG505 DS-SOSIP Env trimer (PDBID:6VI0). (b) VRC07-523LS charge variants were plotted according to the fold change of geometric mean IC80 values and the retention volumes on heparin column chromatography. (c) Pharmacokinetics of VRC07-523LS variants in human FcRn knock-in mice after administering a dose of 5 mg/kg intravenously. (d) PK properties, affinity to heparin, geometric mean IC80 fold changes against a 12-strain panel, and net charge of VRC07-523LS variants. The charge was calculated based on the sequence using Pepstats at https://www.ebi.ac.uk/Tools/seqstats/emboss_pepstats/ (e, f) Neutralizing activity of VRC07-523LS variants against a 208-strain panel.
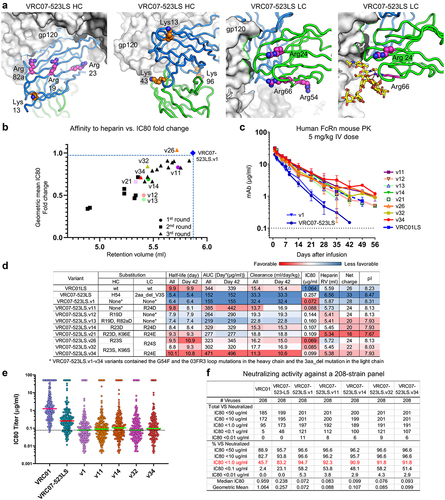
Figure 4. N6LS variants with select Arg or Lys to Asp Gln or Glu substitutions showed improved pharmacokinetics. (a,b) Arg and Lys residues in the variable region of N6LS selected for Asp Gln or Glu substitution were shown in surface representation to the N6LS Fab modeled using VRC01.23-bound BG505 DS-SOSIP Env trimer (PDB ID:6VI0). (c) N6LS charge variants were plotted according to the geometric mean IC80 fold change and the retention volumes on heparin column chromatography. (d) Pharmacokinetics of N6LS variants in human FcRn knock-in mice after injecting a dose of 5 mg/kg intravenous. (e) PK properties affinity to heparin IC80 fold changes against a 12-strain panel and net charge of N6LS variants. (f,g) Neutralizing potency against a 208-strain panel.
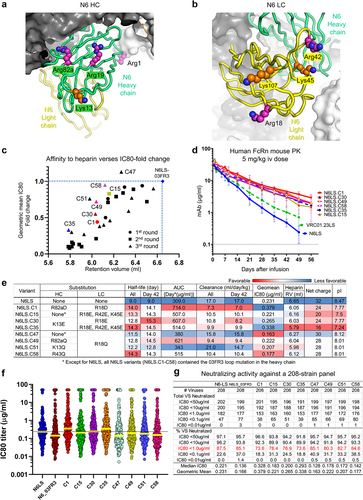
Figure 5. Binding affinities of VRC07-523LS variants to heparin correlated with their binding affinities to FcRn at pH 7.4 when the Fab regions were free to interact with the matrix of biosensors. (a) Ni-NTA biosensors coated with FcRn/β2m were dipped into wells containing VRC07-523LS variants. (b) Ni-NTA biosensors coated with VRC07- 523LS variants were dipped into wells containing FcRn/β2m. (c) FcRn/β2m was passed over VRC07-523LS variants captured by gp120 core immobilized on a CM5 chip. (d) the plot of the KDs measured in (A) vs. the retention volume on harpin chromatography. (e) the plot of the KDs measured in (b) versus the retention volume on harpin chromatography. (f) the plot of the KDs measured in (c) versus the retention volume on harpin chromatography.
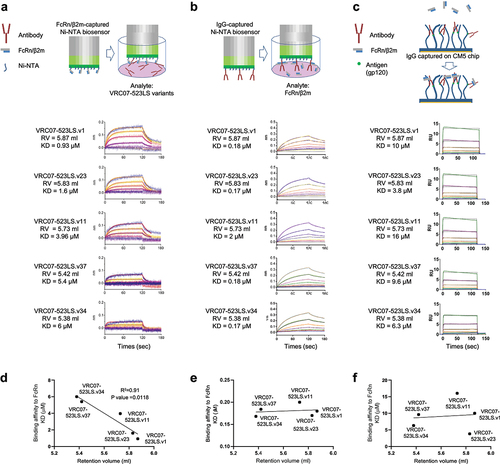
Figure 6. Arg or Lys is highly prevalent among amino acids at select positions in the variable region of human antibodies. (a) the relative frequency of amino acid in the variable region of human antibodies where Arg or Lys is the most or highly prevalent (abYsis server at http://www.abysis.org/abysis/searches/distributions/distributions.cgi. (b) Accessible surface areas were calculated from the structures of eight HIV−1 antibodies. Arg or Lys residues with high ASA and high relative frequency were highlighted in gray background. (c) Arg or Lys residues highly prevalent in human antibodies were shown in stick representation. Close-up view of four Arg or Lys that interact with neighboring Asp via salt bridges.